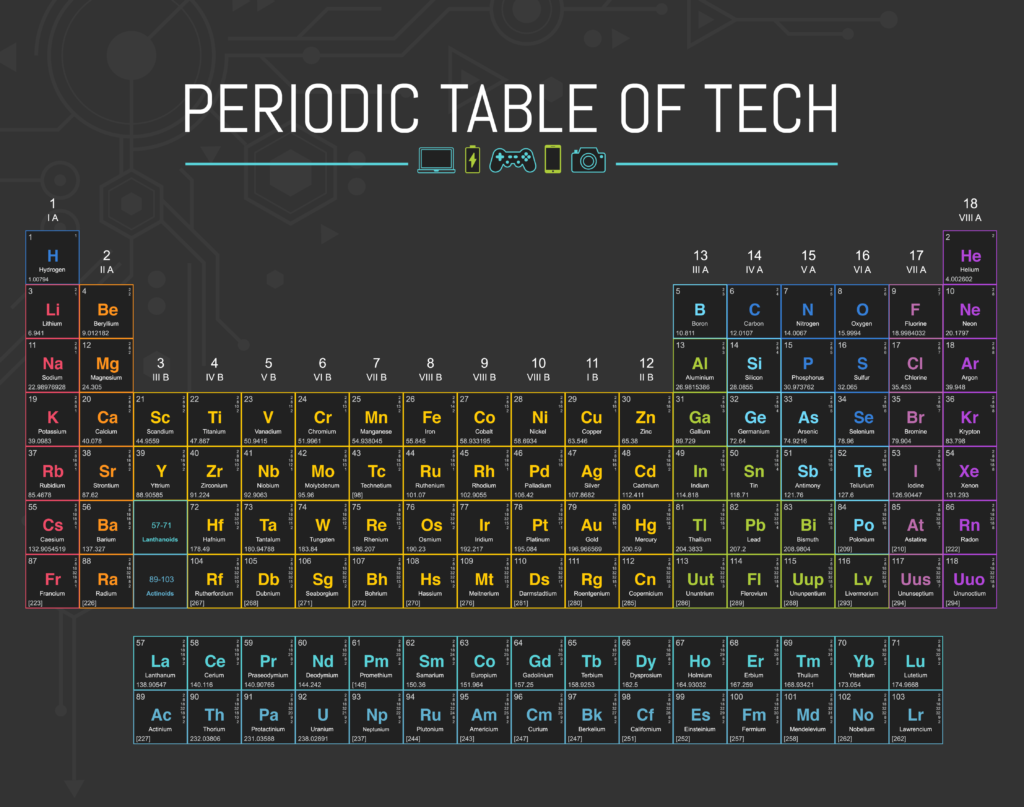
Periodic Table of Tech
SCIENCE, 21 Aug 2017
The Beacon – TRANSCEND Media Service
You’re probably familiar with the periodic table — you may have even memorized all the elements. But do you know how they’re used outside of chemistry class?
Hydrogen (H)
Hydrogen is the most abundant element in the universe, and as its atomic number suggests, it’s pretty important. There’s hydrogen in the air you breathe, the water you drink, and the tech on your desk. Gaseous hydrogen is used to manufacture all kinds of electronics (like silicon computer chips). But it could fuel more than laptops soon — many experts believe hydrogen is the key to clean energy.
Helium (He)
There’s a reason birthday balloons are filled with helium. The noble gas is nonreactive and nontoxic, so it’s unlikely to crash the party. But helium has even cooler applications. With a boiling point of -452 °F, liquid helium is one of the coldest substances in the world. It’s often used to cool down satellite instruments and MRI scanners.
Lithium (Li)
Lithium is the MVP of modern technology. This soft, silvery metal is commonly used to make rechargeable lithium-ion batteries for our favorite devices — cell phones, laptops, digital cameras, and even electric cars. But it’s also used to power the tech we don’t keep on hand all the time. (We’re looking at you, power tools, Roombas, hoverboards, and essentially everything else with a charging port.)
Beryllium (Be)
As a light, hard metal with a sky-high melting point, beryllium is essential in industries that bring the heat. When mixed with other metallic elements, beryllium is used to make springs, gears, cogs, and switches that go inside computers, spaceships, and automobiles. Inception alert: Beryllium alloys are also used to produce the tools that manufacture beryllium-based products.
Boron (B)
It’s a bird, it’s a plane… it’s boron! More often than not, this metalloid can be found in the sky — it’s used in fireworks and rocket fuel. But Boron also has applications at home. Certain compounds are used in laundry detergents and bleaches, as well as mild antiseptics. In the electronics industry, some boron oxides are used to make heat-resistant glass.
Carbon (C)
Carbon is the building block of all life. It can form both graphite (one of the softest materials on earth) and diamond (the world’s hardest substance). It’s used in everyday objects, like black printer ink, but it’s also found in plant and animal cells. Some compounds are deadly, but others are essential components of the air we breathe.
Nitrogen (N)
If you want to get up close and personal with nitrogen, all you have to do is breathe. This omnipresent element makes up approximately 78% of air on Earth! When electronics manufacturers build tech, they use nitrogen gas to create an inert (or nonreactive) atmosphere. This prevents unwanted chemical reactions like oxidation during the manufacturing process.
Oxygen (O)
Element #8 is one of the most important gases on earth — and in the atmosphere. Without the protective ozone layer (which contains oxygen), we’d be exposed to a dangerous amount of ultraviolet radiation. Pure oxygen is most widely used in steel manufacturing. In some factories, it’s blown directly into molten iron to remove impurities.
Fluorine (F)
Been to the dentist lately? You probably heard a lot about flossing and fluoride. Sodium fluoride is a fluorine compound that’s added to toothpaste and drinking water to prevent cavities. But fluorine can clean more than just teeth. In manufacturing, this element is used to refine metals and polish glass.
Neon (Ne)
Neon can take credit for vibrant signs lining the Las Vegas strip. (Fun fact: only red ssigns contain pure neon — you need a mixture of other gases to get the rest of the rainbow.) But, neon is also used in tech production. You can find the gaseous element in TV tubes, power lines and (wait for it) lasers.
Sodium (Na)
Sodium. It’s a dinner table essential and the punchline of every Batman-themed chemistry joke. Aside from its undisputed role in the culinary world, sodium salts are used to manufacture artificial rubber compounds. Conductive rubber coverings are often used in the telco industry to protect wires from electromagnetic interference. This reduces noise in TVs, radios, and audio speakers alike.
Magnesium (Mg)
Since magnesium is lightweight and malleable, it’s used in small portable electronics like phones, laptops, and cameras. Due to increased demand for efficient tech products, magnesium alloys are even replacing plastics — these alloys weigh about the same but are much more durable. Plus, they’re resistant to electromagnetic interference.
Aluminum (Al)
Aluminum is a soft, lightweight metal that can be found in everything from telescope mirrors to airplane parts and electrical transmission lines. This conductive element even has uses in nanoparticle technology. Aluminum is cheap and nontoxic, so when it’s not being used in subatomic science, it’s a major player in the packaging industry.
Silicon (Si)
Silicon is an extremely useful element because it’s an ideal semiconductor. That means it has an intermediate conductivity — it’s not extremely conductive, but it doesn’t stop the flow of electricity, either. Silicon is most commonly used in computer chips, LCDs, and solar cells. Lower quality silicon is often mixed with other metals to create steel, brass, and bronze.
Phosphorus (P)
Steel is a major technology metal — and we likely wouldn’t have as many steel-based electronics or wires without phosphorus. Phosphorus plays an important role in steel production: it increases tensile strength and reduces vulnerability to corrosion. This makes the steel used in our tech a lot more durable, so you can drop your new iPhone 8 without major structural damage.
Sulfur (S)
If you want to find sulfur, you’ve got to go exploring. The characteristically yellow element is found near volcanoes and in deposits deep underground. About 90% oof all sulfur consumed by the U.S. is converted into sulfuric acid. This compound is a key ingredient in the fertilizer that helps grow crops across the country.
Chlorine (Cl)
This familiar element can kill bacteria, so it’s used to keep pools clean. But swimmers beware: liquid chlorine can cause chemical burns in high concentrations. In industry, certain forms of chlorine are used to manufacture silicon, rubber, electrical wiring, and plastic. So, the chemical plays an understated — yet important — role in creating computer chips, TVs, and cell phones.
Argon (Ar)
This colorless, odorless noble gas is best-known for being non-reactive. In fact, argon got its name from argos, the Greek word for “lazy.” The element is commonly used to fill incandescent light bulbs and fluorescent tubes. Manufacturers choose argon instead of normal air because oxygen corrodes the metal filaments inside. Argon increases a light bulb’s lifespan, simply by doing nothing.
Potassium (K)
Pure potassium is super explosive, so it’s rarely found free in nature. (Don’t worry — the healthy stuff in bananas is a potassium compound.) Liquid potassium is often used to regulate temperatures in nuclear power plants by transferring heat from the core reactor toward steam-powered electric generators.
Calcium (Ca)
As the fifth-most-abundant element in the Earth’s crust and the main component of our bones, calcium is a biology superstar. In the tech space, though, this element steps back to let other metals shine. It’s mostly used to remove impurities from iron ore and to produce metal alloys. Calcium with the assist!
Scandium (Sc)
This element may be low-density, but it’s high intensity. Scandium is super strong, making it a great fit for the aerospace industry. Since it can withstand extreme conditions without cracking or melting, scandium is commonly used to construct airplanes. It’s particularly important on exterior parts, like the fuselage. Now there’s a five-dollar word.
Titanium (Ti)
Titanium is a renaissance metal of sorts. It’s as strong as steel but nearly twice as light — and its corrosion resistant. At the turn of the century, Apple released a line of laptops featuring a titanium body. Though the PowerBook G4 was well received, aluminum has proven to be more practical. These days, titanium is primarily used for surgical applications and aerospace construction.
Vanadium (V)
Most of the world’s vanadium is added to steel to make stronger alloys. But a little goes a long way. Even a tiny amount of vanadium is enough to make steel shock and vibration resistant. Once metals are mixed with element #23, they’re ready to endure a variety of volatile environments, from nuclear reactors to combustion engines.
Chromium (Cr)
In industry, chromium is used to create rust-resistant stainless steel. But chromium also has virtual applications — it’s the namesake of the world’s most popular web browser. Google Chrome owes its newfound fame to the Chromium Project, an initiative that ensured everyone’s favorite internet application would be fast, safe, reliable, and accessible.
Manganese (Mg)
Manganese is mainly used in steel production to remove impurities from iron ore. But this element also plays an underrated role in many of our favorite devices. Manganese ferrites (or, fancy magnets) are used in all kinds of electronics, from circuit boards to cable cords. These magnets suppress certain frequencies and reduce interference so that your screens are static-free.
Iron (Fe)
Iron is abundant and inexpensive, giving it some serious popularity points. In fact, it’s the most widely used metal on the periodic table — 90% of all refined metal is iron. It has countless applications and can be found in everything from automobiles to electronics. Although iron is highly corrosive, it’s often alloyed to make more durable metals.
Cobalt (Co)
Silvery-blue cobalt is used to make batteries for rechargeable electronics. Since cobalt improves conductivity, it’s a popular addition to nickel and lithium alloys. With more efficient alloys, manufacturers are able to craft lighter batteries with less environmental impact. Experts predict that the element will see all new applications with the rise of electric vehicles.
Nickel (Ni)
You guessed it. Nickel is the stuff inside our 5-cent piece. It’s mainly used to make small batteries for smartphones and laptops. But it also has a less typical tech application: nickel alloys are used in desalination plants, which convert salt water into drinkable water. What’s the secret to being a desalination star? Corrosion resistance, even in stormy seas.
Copper (Cu)
Copper was one of the first metals ever used by humans. Hundreds of years ago, our ancestors shaped the malleable material into coins. Turns out, they got it right — today, all U.S. coins are copper alloys. Copper has applications in just about every industry, but it’s especially well-suited for electronics. You can thank copper-based coaxial cables for delivering internet and cable TV service.
Zinc (Zn)
About half of all zinc consumed by the U.S. is used to galvanize other metals. With a coat of molten zinc, rust-prone elements are much more resistant to corrosion. Fun fact: galvanized steel is used to construct gigantic structures, like the Golden Gate Bridge. But zinc is more than an additive. Its compounds are used to manufacture all kinds of products, from paint to batteries.
Gallium (Ga)
Gallium is a pretty great stunt double. This element has a similar structure to silicon, so it’s widely used in the electronics industry. You can find gallium in LED lights, cell phones, computers, and TVs. It’s also a great substitute for toxic mercury. Since gallium melts around room temperature, it’s commonly found in medical thermometers.
Germanium (Ge)
Like many semiconductors, germanium is used to manufacture alloys. However, it’s relatively rare, so it’s not in the mix as often as other members of its metalloid family. Most germanium is used to manufacture glass for fiber optics and optical lenses. Since germanium gives glass a high index of refraction, it’s used to create special lenses for wide-angle cameras and microscopes.
Arsenic (As)
Do you feel nervous at the mention of arsenic? Many people do. It’s a common ingredient in pesticides, and it’s been used to poison people, too. (Yikes.) In the tech world, arsenic has a much less sinister purpose: it’s often used to dope silicon in a semiconductor. This allows the silicon to conduct electricity more easily — and makes it more attractive for use in solid-state devices.
Selenium (Se)
Though it’s hard to come by, selenium can be used to manufacture glass and electronic equipment. The solar industry owes a lot to selenium — it was a key ingredient in the first photovoltaic cell. However, it’s not all sunshine and roses: selenium compounds are toxic. In fact, plants grown in soil that contains selenium may become poisonous.
Bromine (Br)
Bromine is commonly used as a flame retardant in plastics — particularly in circuit boards and wire casings. These additives are used to prevent electrical fires. However, research suggests that these chemicals are toxic. Lately, there’s been much debate over whether handheld electronics even need to be fire-proofed. Apple weighed in by replacing BRFs with safer alternatives.
Krypton (Kr)
Krypton doesn’t just spell trouble for Superman. Even outside the city of Metropolis, some isotopes of krypton are radioactive. Safer compounds of the clear gas are often combined with xenon to produce energy-saving light bulbs. These krypton-filled bulbs produce brighter light and last longer than their halogen-filled counterparts.
Rubidium (Rb)
Though rubidium is usually restricted to the confines of a research lab, it has been used to manufacture photoelectric cells. Generally, a photocell is any device that uses light to generate an electric current. These cells can power a variety of electronics including alarm systems, automatic door openers, and solar batteries.
Strontium (Sr)
Certain isotopes of strontium are too dangerous to touch — Strontium-90 gives off enough beta radiation to generate electricity for space vehicles. But, in safer forms, the element can be used to produce the Cathode Ray Tubes (CRTs) used in old-school TV screens and computer monitors. Talk about an element that can do both.
Yttrium (Y)
Need a durability boost? Just add yttrium. Yttrium oxides are used to strengthen metal alloys and to make glass heat and shock resistant. This is great news for professional photographers, selfie enthusiasts, and everyday smartphone users who rely on clear, durable camera lenses.
Zirconium (Zr)
If this name sounds familiar, you’re probably thinking of cubic zirconia, a zirconium oxide that crystallizes as a diamond-like synthetic gemstone. Tech-wise, zirconium is primarily used in nuclear power stations since it’s resistant to both chemical corrosion and heat. This metal works particularly well in nuclear fuel rods to help facilitate reactions.
Niobium (Nb)
Niobium alloys are super strong, so it’s no surprise that they’re found in jet engines. The element is primarily used to strengthen other metals, and its ability to work well with others gives it a wide range of applications. For example, niobium-titanium alloys are used to build MRI magnets. This just proves that sometimes two elements are better than one.
Molybdenum (Mo)
No movie night is complete without molybdenum — the shiny metal is a key ingredient in manufacturing LCD screens. Compared to more outdated cathode-ray tubes, LCD technology offers dramatically improved picture quality. And since LCDs are less bulky than CRTs, they’re often used in modern flat screen TVs, tablets, computers, and smartphones.
Technetium (Tc)
Technetium was the first element to be produced synthetically. This artificial metal has never been found on earth (though it has been seen in stars). For now, we’re happy to keep technetium light years away — the radioactive element is toxic. Technetium can be added to steel to protect it from corrosion. However, this alloy has limited uses since direct exposure is so dangerous.
Ruthenium (Ru)
When mixed with other metals, ruthenium is used to manufacture chip resistors. Chip resistors help regulate electrical currents in compact circuit boards. They can be used to either maintain the electrical current or drop the voltage down lower. Engineers are working to develop more efficient resistors so that they can be used in our smallest handheld devices.
Rhodium (Rh)
Rhodium is primarily used in to create catalytic converters for cars, trucks, and other automobiles. (No, they’re not limited to Cadillacs.) Though catalytic converters aren’t as exciting as rims or spoilers, they’re a critical component of every car. These devices control vehicle emissions by converting toxic gas exhaust into less harmful pollutants.
Palladium (Pd)
Palladium is commonly used in the electronics industry to make ceramic capacitors for laptops and cell phones. In this type of capacitor, palladium sheets are insulated by ceramic layers. These versatile devices can be produced in a variety of sizes and voltages, so they’re found in everything from circuit boards to robots.
Silver (Ag)
As the most conductive metal on the periodic table, it’s no surprise that silver is expensive. Though it was historically used in jewelry, coins, and luxury items, silver is now primarily used in electronic tech. The shiny metal can be found in tracking devices, solar panels, LED lights, and TVs. If it has an on/off switch, chances are it contains silver.
Cadmium (Cd)
The vast majority of cadmium is used to produce rechargeable batteries. The element’s low melting point makes it a valuable ingredient in solder alloys as well. Solder alloys are designed to seal two metals together — usually electrical components or pipes. Unfortunately, cadmium is a known carcinogen. Since direct exposure is dangerous, many organizations have eliminated its use.
Indium (In)
Indium is a soft silvery metal named after the indigo-colored line in its spectrum. It’s often used to produce indium tin oxide (ITO), a key ingredient in touch screens. Touch screens need a conductive material like indium, which can detect changes in its electric field. (Yep, your fingers hold an electric charge.)
Tin (Sn)
Humans have mined and used tin for thousands of years. Our ancestors mixed tin and copper to create bronze, which they then used to craft statues, weapons and tools, jewelry, coins, and more. (Want a mini history lesson? See also: the Bronze Age.) Today, we use tin to create powerful magnets and superconductive wire.
Antimony (Sb)
Antimony is a poor conductor of electricity, but still has an important role in many of the electronics we use. The semi-metal is used to manufacture batteries, diodes, and cable sheathing, which can make devices more efficient. It can also be added to compounds in order to make them fire-resistant. Thinking of making a superhero suit? Antimony might not be a bad addition.
Tellurium (Te)
Small amounts of tellurium can make big improvements to metal alloys. For example, just a little tellurium increases tin’s hardness and makes copper and stainless steel easier to work with. Tellurium compounds can be used to manufacture thermoelectric devices, but these power generators aren’t quite as efficient as their competitors.
Iodine (I)
Seaweed isn’t just for sushi anymore. Kelp can be dried, burned and processed to create iodine. For decades, this element has been used to produce photography film. But more recently, iodine is used to manufacture LCDs for smartphones and HD TVs. It’s used to make polarizing films, which increase the visibility of images on your screen.
Xenon (Xe)
Like many other noble gasses, xenon is widely used in lighting. It’s built a reputation as the best and brightest when it comes to camera flash technology. However, you’re probably not carrying one around in your pocket. Most smartphone cameras rely on LED lights because they’re smaller and cheaper than xenon flashes. That hasn’t stopped companies like Fotopro from trying, though.
Cesium (Cs)
Late to class? Blame cesium. This golden metal fuels the world’s most accurate atomic clock. Using microwave emissions from cesium atoms, the clock (affectionately named NIST-F2) defines the exact length of a second and sets the standard for all other timepieces. It’s used to synchronize time across cell phone networks, power grids, GPS satellites, and the internet.
Barium (Ba)
Barium compounds are toxic, so they aren’t as popular as many other alloys. However, this dense metal can be used to manufacture glass. Even if you can’t see it, glass is a fundamental component in all kinds of technology. The material has applications in virtually every industry — from solar energy panels and TV screens to medical devices and fiber-optic cables.
Lanthanum (La)
Lanthanum is so soft that it can be cut with a knife, so the metal is not particularly useful on its own. However, its alloys are. Car companies like Toyota and Nissan use lanthanum to make rechargeable batteries for their hybrid cars. Beyond the auto industry, the U.S. military uses lanthanum to produce defense technology, like night vision goggles.
Cerium (Ce)
Be careful around pure cerium — it’ll spark if it comes in contact with another sharp object. That’s why it’s widely used in lighter flints. Cerium is much tamer when combined with other metals, so its alloys are used in a variety of electronics, from low energy light bulbs to flat screen TVs. Some cerium compounds can also help produce, polish, and remove color from glass.
Praseodymium (Pr)
Praseodymium is a silver, moderately toxic element that’s used to manufacture high-intensity magnets. These magnets are an important power source in green electronics, like hybrid car engines and wind turbines. But this rare earth metal has both brawn and beauty. Its compounds produce yellow glass and synthetic green gemstones.
Neodymium (Nd)
This element is primarily used to create super-strong permanent magnets. These magnets can be found in all kinds of electronics, from computer hard drives to motors. Neodymium can also add special properties to glass. It can be used to make fiber optic cables (which transmit high-quality TV and internet service) or to block harmful light rays in welding goggles.
Promethium (Pm)
Promethium is a radioactive metal with no stable isotopes, so don’t get too close. Though most promethium is reserved for research, it can be a source of energy in small electronics that need a dependable power source, like pacemakers. Scientists believe that promethium’s radioactive decay could be converted into electricity to power nuclear batteries.
Samarium (Sm)
Hard, heat-resistant cobalt is the perfect partner in crime for a soft silvery metal like samarium. Together, these elements make strong magnets. Samarium can also be used to produce the cathode ray tubes inside old-school computer displays and TV screens. CRT monitor sales peaked at the turn of the century, but have rapidly declined to make way for higher quality LCD technology.
Europium (Eu)
Europium is a soft, reactive metal with a propensity for spontaneous combustion when it gets too hot. These properties make it a great nuclear fuel source. But europium’s radioactivity makes it useful even beyond the power industry. Fun fact: europium compounds are added to Euro banknotes as anti-counterfeit measures. On genuine notes, the europium glows red under UV light.
Gadolinium (Gd)
This element plays a minor (but important) role in all kinds of places. In the electronics industry, gadolinium can be found in CDs and computer memory. In manufacturing, the magnetic element is added to alloys to make hard metals more workable. Most importantly, gadolinium is used in the medical field to enhance MRI images. This helps doctors accurately detect cancerous tumors.
Terbium (Tb)
This little-known element can be found in all kinds of technologies, from fiber optics to smartphone screens. However, terbium is primarily used to stabilize fuel cells and manufacture phosphors. A phosphor is a solid material that can emit light when exposed to radiation. Terbium phosphors typically give off a green light — you’ll find them in TVs and computer monitors.
Dysprosium (Dy)
Like many other lanthanides, dysprosium is too temperamental to have widespread applications on its own. But, that changes when it’s combined with other elements. A special dysprosium-based cement is used to safely cool nuclear reactor rods. And when dysprosium is combined with other rare earth metals, it can be used to make laser materials.
Holmium (Ho)
Recent research has turned up some exciting applications for holmium. According to IBM, the average hard drive uses up to 10,000 atoms to store one bit of data. However, researchers have proven that it’s possible to store data on a single atom. Using holmium’s magnetic field, IBM scientists invented the world’s smallest magnet. For now, it’s just a prototype, but it holds huge promise.
Erbium (Er)
Love super-speedy internet? We owe it to erbium. This rare-earth element emits light at wavelengths commonly used in fiber-optic signal transmission. So, erbium is most commonly used to produce signal amplifiers for long-distance fiber-optic cables. Fiber-optic technology is popular because it uses light (not electricity) to transmit lots of data, faster than aging coaxial cable networks.
Thulium (Tm)
Thulium can be combined with durable yttrium to create high-temperature superconductors. And though the element is slightly toxic, certain isotopes can be used in x-ray equipment. There’s just one issue: thulium costs a pretty penny. The expensive metal is also being considered as a potential energy source, but for now, it has little practical use beyond research.
Ytterbium (Yb)
Ytterbium is a relatively rare, volatile metal. It can be used in portable X-ray devices and as a dopant in a variety of materials. Dopants are intentionally added to semiconductors in order to alter their electrical properties. In this case, ytterbium-doped glasses have a simple electronic structure and are well-suited for fiber-optic cables.
Lutetium (Lu)
Lutetium is the last of the lanthanide metals. It’s high-melting and incredibly dense, but it isn’t very useful in industry. Lutetium-176 can help us determine the age of meteorites. However, outside of astronomy, there aren’t many uses for lutetium. For now, we’ll keep this silvery metal locked in the research lab — especially since we aren’t sure just how toxic it is.
Hafnium (Hf)
Hafnium is so similar to zirconium that it’s often difficult for scientists to tell them apart. Like its identical twin element, hafnium is useful in the nuclear power industry. But it’s more commonly used as an electrical insulator in microchips. By ensuring that these chips conserve energy and run more efficiently, hafnium helps us make smaller microchips for portable electronic devices.
Tantalum (Ta)
About half of all tantalum is consumed by the electronics industry. The silvery metal is resistant to corrosion, so it creates efficient capacitors for mobile phones, laptops, and gaming consoles. (Capacitors are the magical devices that keep your electronics charged.) Since tantalum oxides are insulating, they’re also used to coat other metals.
Tungsten (W)
There’s no other way to say it, tungsten is tough. The super-dense element is almost impossible to melt — in fact, it has the highest melting point of all metals. As you might guess, tungsten is important for jobs like welding and metal-working. But you can also find tungsten in your everyday electronic devices. Since it can withstand erosion, tungsten is commonly used to make electrical circuits.
Rhenium (Re)
Rhenium is one of the rarest metals around. When it is found, it’s usually combined with tungsten to create an extremely heat-resistant alloy. Rhenium’s durability makes it useful in x-ray machines, where it’s constantly exposed to radiation. But, the material is also great at passing electrical currents from one conductor to another. Which devices need electrical conductors? Only all of them.
Osmium (Os)
Due to its high density, osmium is a great material for making electrical contacts. Electrical contacts are the building blocks of modern electronics — they help electricity flow through devices. A good contact reduces friction and helps ensure that electricity moves efficiently. Since osmium is conductive and strong enough to carry high voltage currents, it’s the perfect element for the job.
Iridium (Ir)
The most corrosion-resistant metal on earth is actually an element from outer space. Scientists believe that iridium was scattered across the globe when a giant meteor collided with earth millions of years ago. Despite its alien origins, iridium is a highly-coveted alloying agent. The hard, durable, heat-resistant metal is often used to produce spark plugs.
Platinum (Pt)
They don’t call it “going platinum” for nothing. In terms of tech, this element is one of the best. Platinum is a great conductor of electricity, so it’s used to make fiber optics, LCD displays, and even pacemakers. The precious metal is also adored by the auto industry. About half of all platinum is used in catalytic converters, which clean up car emissions and improve fuel efficiency.
Gold (Au)
Watches. Teeth. Olympic medals. Gold can be used to make just about anything. The rare yellow metal is an excellent conductor of electricity, so it’s a key ingredient in high-end electronics. Despite its industrial utility, gold is most popular for its looks alone — about 78% of gold is used to manufacture jewelry.
Mercury (Hg)
Mercury is the only metal that’s liquid in its natural phase. But it’s better to admire at a distance — mercury is toxic in high doses. For centuries, it was used in common household items like batteries, fluorescent lights, and thermometers. In order to reduce the risk of mercury poisoning, the World Health Organization has taken action to phase out non-essential mercury products.
Thallium (Tl)
Thallium is so dangerous that it was historically used as rat poison. But rodents can relax — in recent years, thallium-based poisons have been banned in the United States. The electronics industry uses thallium sulfide (a mixture of thallium and sulfur) to produce photoelectric cells. These cells are particularly unique because their conductivity increases when exposed to certain types of light.
Lead (Pb)
Commercial use of lead in paint and other household products has become less common since we figured out what lead poisoning is. (Makes sense.) Lead is still often found in car batteries and electrical cable sheathing, so those who frequently come in contact with those objects — like mechanics or construction workers — take special precautions to avoid exposure.
Bismuth (Bi)
This metal is a fire station favorite. Though it’s too brittle to be used on its own, bismuth is often mixed with other elements to make alloys. When bismuth is alloyed, the finished product picks up some of the metallic element’s best properties, like its low melting point. Since these alloys can withstand high heats, they’re often used in smoke detectors and fire extinguishers.
Polonium (Po)
Polonium is rare and highly radioactive. Though it can be used as a heat source for space equipment, its half-life is too short to generate long-term energy — some polonium isotopes have a half-life of less than a second. Polonium does have a unique purpose: it helps reduce static. Today it’s mostly used to eliminate static in manufacturing processes for paper, plastic, and metal.
Radon (Rn)
Radon exists naturally in the earth’s soil, but it can be extremely dangerous. As radon decays, it produces other radioactive isotopes like polonium and lead. Exposure to radon (and its daughter elements) has been linked to lung cancer. It was once used to target cancerous cells via radiation therapy. However, today doctors use more efficient isotopes to treat cancer.
Thorium (Th)
That’s right, this metal was named after the hammer-toting mythological god, Thor. Though thorium compounds can produce camera lenses and solar panels, it’s found in nuclear reactor fuel. It’s predicted that the thorium metal in the earth’s crust could produce more fuel than both uranium and fossil fuels.
Uranium (U)
Radioactive uranium is a well-known fuel source for nuclear power plants. This reactive metal releases energy when its atoms split during a process called nuclear fission. Just one pound of uranium produces as much fuel as 1500 tons of coal. Accidental exposure to materials like uranium could have deadly consequences for humans, animals, and the environment.
Plutonium (Pu)
Plutonium is named after Pluto. This radioactive element has extraterrestrial applications — it’s used to generate electricity for space vehicles that travel so far from the sun that they can’t use solar power. Due to its explosive properties, it was used in the first atomic bomb and is still used in nuclear weapons.
Americium (Am)
Though this radioactive element is toxic, it can help save lives. Americium is a key ingredient in smoke detectors. The element emits a constant stream of alpha particles, and if smoke interrupts the flow, it triggers the alarm. Americium ionization detectors react to the tiny smoke particles produced by fast-burning fires faster than photoelectric alarms can.
Curium (Cm)
Curium is mainly used for scientific research. However, the transuranium element has some interesting properties. Just one gram of curium produces three watts of thermal energy, outpacing radioactive plutonium. Curium can be used to generate energy for space vehicles. In fact, a curium battery was used to fuel instruments on the Mars Pathfinder mission.
Californium (Cf)
Californium is a completely synthetic element that does not exist in nature. The radioactive metal was discovered by chemists at the University of California Berkeley, and it’s named after the Golden Bears’ home state. For now, engineers are working on timed neutron detectors, which use californium isotopes to detect landmines.
Go to Original – fios.verizon.com
DISCLAIMER: The statements, views and opinions expressed in pieces republished here are solely those of the authors and do not necessarily represent those of TMS. In accordance with title 17 U.S.C. section 107, this material is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. TMS has no affiliation whatsoever with the originator of this article nor is TMS endorsed or sponsored by the originator. “GO TO ORIGINAL” links are provided as a convenience to our readers and allow for verification of authenticity. However, as originating pages are often updated by their originating host sites, the versions posted may not match the versions our readers view when clicking the “GO TO ORIGINAL” links. This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond ‘fair use’, you must obtain permission from the copyright owner.