The Gene Drive Dilemma: We Can Alter Entire Species, but Should We?
SCIENCE, 13 Jan 2020
Jennifer Kahn – The New York Times
A new genetic engineering technology could help eliminate malaria and stave off extinctions — if humanity decides to unleash it.

A Drosophila melanogaster fruit fly engineered with a gene drive that caused it to have red fluorescent eyes.
Craig Cutler for The New York Times
8 Jan 2020 – One early summer evening in 2018, the biologist Anthony James drove from his office at the University of California, Irvine, to the headquarters of the Creative Artists Agency, a sleek glass-and-steel high-rise in Los Angeles. There, roughly 200 writers, directors and producers — many of them involved in the making of science-and-technology thrillers — were gathered for an event called Science Speed Dating, where James and other scientists would explain their work. The sessions were organized, James told me, “in hopes of getting the facts at least somewhat straight.”
Attendees were assigned to different groups, so each scientist had just seven minutes to describe his or her work to one group before running to the next room and starting over. “There were a lot of stairs, so I would get really out of breath,” James recalled. “I would arrive panting.” He also felt a bit overwhelmed. There were executives in expensive suits, young men and women looking unaccountably dressy in ripped jeans and, according to James, a disconcerting number of people wearing hats. Few, if any, had a deep knowledge of genetics; one participant in particular kept referring to “the dark genome,” as though that were a thing. “I had to tell him, ‘Real geneticists don’t usually talk that way,’ ” James said.
James began his presentation with a brief overview of mosquito-borne diseases like malaria and Zika. Then he turned cautiously to talking about his own area of scientific expertise: an obscure but powerful invention known as a gene drive. James began by noting that two brown-eyed human parents can sometimes produce a blue-eyed child, though only if both parents carry a copy of the recessive gene. A gene drive, he explained, was a tool that in some species could turn such events into a near certainty. For one thing, it guaranteed that a particular gene would be inherited, even if only one parent had it. And it would automatically insert the chosen gene into both copies of the offspring’s DNA, effectively turning a recessive trait into a dominant one. That alone, James explained, “lets you change the odds, so you get blue eyes 99 percent of the time.”
What made the gene drive truly strange and remarkable, though, was that it didn’t stop with one set of offspring. Generation after generation, it would relentlessly copy and paste the gene it carried, until it was present in every descendant. “For most of the people in the room, you could tell it was the first they’d heard of this,” James recalled. “You could see their eyes getting big.”
This mattered, James explained, because it allowed you to change not just a single creature but — potentially — an entire population, and quickly. A few months after the technique was discovered in 2014, James engineered two mosquitoes to carry a gene drive that was tied to a gene for red fluorescent color that would target the mosquitoes’ eyes. He then put each into a box with 30 ordinary purple-eyed mosquitoes. As the mosquitoes bred, they produced offspring: roughly 3,900 after two generations. (Mosquitoes lay a lot of eggs.) Under the normal rules of inheritance, there should have been an equal number of red-eyed and purple-eyed mosquitoes. Instead, when James opened the boxes to check on the offspring, all but 25 of the 3,900 mosquitoes had red eyes.
Leigh Dana Jackson, a producer who was adapting a fantasy novel called “The Fifth Season” for television, was one of the people who saw James’s talk. “I was fascinated by the fact that this was already real,” he told me. It wasn’t hard to imagine the Hollywood version of the gene-drive story: the rogue scientist determined to destroy global agriculture by breeding an unstoppable army of insects (working title: “The Plague”); the corrupt corporate titan who, warned that gene drives can mutate wildly, silences the researcher, determined to see a return on his investment.
So far, at least, the reality is less lurid. Gene drives have yet to be tested outside the lab, and even the most developed project to date — the anti-malarial gene drive in Anopheles mosquitoes — won’t be widely available for at least another five years. But many scientists and public-health experts believe that the benefits could be significant. Besides combating malaria, gene drives could be used to alter, or even eliminate, other disease-causing insects, from the sand flies that transmit leishmaniasis to ticks that carry Lyme disease in the United States. (Because the spread of a trait happens over generations, a gene drive works best in species that reproduce quickly, like insects and rodents, rather than in, say, elephants and people.) They could also be used to protect endangered species. In the Galápagos, environmental groups like Island Conservation and the International Union for Conservation of Nature have explored using an “all-male” gene drive — one that results in only male offspring — to eliminate the rats that are decimating the native bird and turtle populations, which are currently managed with poisoned bait. And among agricultural researchers, gene drives have been floated as a strategy for combating invasive crop pests, like the spotted-wing fruit fly, without pesticides.
For now, though, much of the potential of gene drives is still highly speculative, and there are an appreciable number of unknowns. In a cautiously supportive 2016 report, the National Academy of Sciences warned that “considerable gaps in knowledge” remain around gene drives’ ecological and evolutionary impacts. Could a gene drive stop one virus only to open the way for another, more virulent one? Could it jump from one species to a related one? What would be the environmental effects, if any, of altering the genes of entire species? How about eliminating a species entirely?
For the speed daters, at least, those questions seemed to resonate. “You’re thinking, O.K., if you’re talking about a benevolent scientist using this, great,” Jackson told me later. “But what about the alternative?” During the Q. and A. session afterward, Jackson recalled, one attendee seemed especially alarmed, pressing James about how gene drives could be used in the hands of an unscrupulous foreign power. “He was the Jeff Goldblum character!” Jackson joked.
Hollywood, of course, isn’t a precise litmus test for how a new technology is likely to be received by the public. But it’s also not a bad approximation. Like screenwriters, most of us tend to gravitate toward the more extreme examples of a technology’s potential, its ability to save the world or to destroy it. Gene drives seem almost tailor-made to tap into our worst fears: a powerful, invisible technology that spreads of its own accord, carrying out a fundamental transformation of nature. It’s a situation that practically invites us to imagine evil corporations on the move, or secret military experiments running amok.
As Alta Charo, a professor of law and bioethics at the University of Wisconsin, Madison, says of our genetic-engineering capability, “At a very instinctive level, there’s a sense that these are things humans are not supposed to be doing.” She went on: “In the years when gene drives weren’t very effective, they also weren’t very risky. It’s a funny situation: When the technology is weak, you want to make it better. But when that happens, suddenly all these things you were imagining actually become possible. You can make that new animal, or you can wipe out that whole species. And if doing that turns out to have been a bad idea, it means you’re going to have to deal with the consequences.”
One paradox of scientific breakthroughs is that they can seem at once flukish and inevitable. Researchers may toil incrementally for decades, unsure whether their work will ever lead to anything, only to find that they have suddenly developed a technology that raises all kinds of real-world questions to which no one has the answers.

Three Drosophila melanogaster fruit flies engineered to carry a gene drive. The blue-eyed fly is an early attempt to study the use of a gene drive to create sweeping changes in a fly’s DNA. Craig Cutler for The New York Times
Gene drives emerged in just this way: Malaria researchers spent almost two decades trying to create gene drives with the aim of curbing disease, but no one was able to make them work very well. In his 10 years of attempts, James managed to increase the chance that a trait would be inherited by just 1 or 2 percent. Then, almost by chance, a new development transformed gene drives from a backwater science into a vanguard technology.
In 2014, Valentino Gantz, a 30-year-old graduate student in biological sciences at the University of California, San Diego, was struggling to finish his thesis — an abstruse project about wing development in flies. Fruit flies typically have five large veins in their wings, but those with a particular genetic mutation will grow only four. Gantz had spent six months trying to see if the gene controlling the missing vein in one species of fly would do something similar in another.
The project wasn’t going very well. Genetic engineering is finicky work. Gantz started by fabricating a gene that caused the mutation for the missing vein; then he used a hair-thin glass needle to inject that into fly eggs, each the size of a grain of sand. The process worked, according to Gantz, roughly one time in a hundred. Even then, the resulting fly got only a single copy of the defective gene, and it took two to produce the missing vein. Because the gene was recessive, moreover, there was no way to tell just by looking which adult fly received it. Gantz’s only option was to blindly engineer, and crossbreed, scores of flies, in hopes that two carrying the recessive mutation would eventually find each other and mate. But so far, despite hundreds of hours of work, Gantz had failed to generate even a single mutated fly.
Then he had a radical idea. Gene editing relies on a tool called Crispr, which is itself a gene, originally found in bacteria. With some modifications, Crispr can be used to cut and paste pieces of DNA almost like a word processor. Gantz decided to use Crispr to insert not just a particular altered gene but also a copy of the Crispr editing tool itself: The two would be tied together. This created a kind of serial copy-and-paste function; the altered gene would be inserted into both copies of the DNA, rather than just one, effectively turning a recessive trait, like a missing vein, into a dominant one.
The ability to generate double-recessives would have been a valuable lab tool in itself. But there was more. Put engineered DNA into most cells — skin cells, brain cells and so on — and it will create a one-time change in the individual who receives those cells. The exception is if the altered DNA is put into a germ cell: the cells that turn into sperm and eggs. Gantz and his thesis adviser, Ethan Bier, a professor of cell and developmental biology at U.C. San Diego, decided to try putting the Crispr editing machinery into a germ cell, along with the gene it was originally tied to. This, they hoped, would cause it to copy itself relentlessly into every egg a fly laid, essentially guaranteeing that a trait would be inherited and keep spreading with each generation.
Scientific research is full of tantalizing ideas that don’t pan out, and Gantz and Bier suspected that this might be one of them. “I would say pretty much everyone assumed it wasn’t going to work,” Gantz told me. “The feeling was more like, ‘Hey, why not try it?’ ”
Gantz bred a round of fruit flies that had been engineered to carry both the Crispr machinery and a single copy of a recessive gene for yellow coloration. As the first batch was maturing, Gantz peered into the vials, only to see what appeared to be ordinary brown flies. “I told Ethan, ‘It didn’t work,’ ” Gantz recalled. “I was really crushed.” Bier advised him to wait a bit longer, to let the pupae develop. When Gantz looked again the next morning, he saw one fly that was yellow, and then another, and another. “That’s when I started jumping,” he told me.
It still wasn’t clear, though, whether the copy-and-paste process would continue in the flies’ offspring, so Gantz prepared a second round of flies, made by breeding the first-generation yellow flies with ordinary brown ones. At the same time, he and Bier began writing a paper on their discovery, with a plan to add the inheritance results at the last minute if the experiment ended up working out.
The second generation of flies was due to mature on Dec. 28, and as the date approached, Bier and Gantz spent hours discussing the possible results. On Christmas Day, Bier invited Gantz to come over to his house for lunch. The two talked for most of the day, hashing out details related to the paper and wrestling with the ethical and safety issues that their breakthrough might raise. “That afternoon, we went through all the worries,” Gantz recalled. What would happen, for instance, if some of the engineered flies got out and began spreading their mutation in the wild? There was also the question of whether such a tool could be used as a weapon — say by sabotaging the pollinators that support agriculture, or by altering the genes of innocuous wild insects so they could transmit disease. “At one point, we were thinking, Should we even publish this?” Gantz remembered.
As a check, Bier phoned another scientist, Hugo Bellen, who is well known for his work in fruit-fly genetics. As Gantz recalled it, Bellen told Bier, “ ‘When you have a result, it’s unethical not to publish it.’ So we decided to go ahead.”
Read more about Crispr and the possibilities of gene editing technologies.
Gene drives are the latest in a string of new genetic tools designed to help us improve our environment or our lives. But while we’ve become adept at making technological breakthroughs, we’ve mostly failed to create real forums for talking about them. “There are big philosophical questions that have been raised at various points but never answered,” says Ben Hurlbut, a historian of science at Arizona State University. “Like, What does a good future look like, and who gets to decide?”
With populism growing and fewer people willing to trust the judgment of regulators and scientists, the rhetoric around complex innovations has become increasingly polarized, with both sides stuck fighting a high-stakes battle for public opinion. Many scientists I spoke to cited the introduction of genetically modified foods as a turning point; the backlash effectively crippled the field. “The level of organized skepticism around genetically engineered foods was a whole new phase,” Charo told me. For one thing, the process — which involved, for example, grafting a flounder gene into a tomato to make it frost-resistant — struck most people as vaguely creepy. Perhaps more distressing, though, was that the technology was controlled primarily by the global agricultural giant Monsanto, which not only held the patents to the new seeds but also quickly began an aggressive global marketing campaign to persuade farmers to switch to its trademarked seed lines.

An Anopheles stephensi mosquito equipped with a gene drive and engineered so that it cannot transmit malaria.
Craig Cutler for The New York Times
“With genetically engineered foods, in the earliest years, Monsanto really set the context,” Charo says. “And it was a mess. Their financial interest in the intellectual property and their regulatory interest in making sure these products were able to come to market got conflated with the science, so nobody was willing to trust the kind of research they were doing. The end result was that all G.M.O. research got tainted.”
Todd Kuiken, a researcher at the Genetic Engineering and Society Center at North Carolina State University, says that “it was basically a lesson in how not to do things.” But, he pointed out, the “Monsanto Mistake” also alerted researchers to the need for a more transparent and collaborative approach. With gene drives, groups like Target Malaria, a nonprofit research consortium administered by Imperial College, London, and funded in part by the Bill and Melinda Gates Foundation, have stressed that the deployment of modified mosquitoes in Africa should be “an African decision.” Local and national governments would work with regulatory organizations like the United Nations and the World Health Organization, which have proposed frameworks for testing and releasing genetically modified mosquitoes. In the United States, recent developments in genetics, including gene drives, have created a boom market for ethicists, as well as for so-called engagement specialists, who have the unenviable problem of figuring out how to get people to be genuinely thoughtful about a confusing and highly technical area of research.
So far, at least, the process has been rocky. At the United Nations Convention on Biological Diversity in Sharm el Sheikh, Egypt, in November 2018, a coalition of activist groups compared gene drives to the atomic bomb and accused researchers of using malaria as a Trojan horse to cover up the development of agricultural gene drives for corporate profit. Scientists working with the Gates Foundation, in turn, accused activists of trying to hijack the meeting and dismissed calls for a global research moratorium. “The report I’ve had so far is that there’s been a lot of yelling,” one policy expert told me glumly.
Natalie Kofler, who attended the conference and runs a global initiative for the responsible development of genetic technologies, described the atmosphere as “pretty raw.” Kofler told me that nongovernmental organizations like Target Malaria tend to be dismissive of activists’ claims, which in turn feeds into the activists’ sense of not being heard. “There’s a general idea that these groups aren’t scientific, so their arguments are less valid,” she says. On the other hand, she went on, some activist groups have started behaving in ways that are deliberately inflammatory. A handful of small NGOs, collectively known as SynBioWatch, have taken to describing gene-drive researchers as a cabal and using tactics more typical of political misinformation campaigns (filing FOIA requests for thousands of emails, then publishing the result — a banal mix of research chitchat and conference planning — framed as a “trove” of hidden evidence). Several scientists I spoke with described feeling embattled; one had recently been subject to a public-information request for her university emails, filed by an activist who also made derogatory remarks about her and her child. Another described seeing online forums hijacked by groups seeking to conflate gene drives and G.M.O.s. Over the summer, a Canadian organization known as ETC Group released a deck of cards showing the potentially alarming uses to which gene drives could be put. One card featured a cartoon image of a gene-drive honeybee that could supposedly be controlled with a beam of light; another showed an aggressive-looking anti-malarial mosquito with the vague but ominous warning, “Covers up the real story of gene drives.”
Jim Thomas, a co-executive director for ETC Group, acknowledges that the honeybee example was highly speculative, though he noted that someone filed a patent for such an idea. But he defends it as a necessary counter to what he regards as a misleading focus on “high-profile savior applications” like anti-malarial and conservation efforts. “The discussion from the beginning has been framed around those best-case scenarios,” Thomas says. “And there’s a sort of unwillingness to discuss where this technology will go from there. Our sense, at least, is that this is an agricultural technology: that it will have its biggest application in agriculture and the food system. And it’s also a technology that’s of interest to the military. But there’s no discussion about that.”
Playing to fears around worst-case scenarios can be a powerful tactic. Dietram Scheufele, who studies scientific and political communication at the University of Wisconsin, Madison, says that scientists are generally much worse than activist groups at shaping public opinion, in part because they tend to rely on logical reasoning and facts, while activist groups are more likely to tap into unconscious values and emotions — like using the term “Frankenfoods” to describe G.M.O.s. “It’s really a brilliant bit of framing,” Scheufele says. “The message is: ‘Science is putting together two things that don’t belong together. And that stuff gets out of control and out of the lab, and it’s all because of scientific hubris.’ And then you have the scientific response, which is someone saying: ‘Actually, that’s not quite right. Let me explain this complicated thing to you. … ’ ”
For the layperson, sorting through such disparate viewpoints can be confounding. “If you talk to most of the members and delegates at the conference, they haven’t even heard of gene drives before now,” Kuiken says. “And then they hear people saying that we’re either going to end malaria with this or else it’s going to destroy the planet and hand control to Big Ag. I mean, what do you do with that?”
The first place a gene drive will most likely be used is the landlocked West African country Burkina Faso. Abdoulaye Diabaté is a vector biologist and the head of the medical entomology laboratory at the Institut de Recherche en Sciences de la Santé, in the country’s second-largest city, Bobo-Dioulasso. He told me that in Burkina Faso, malaria-carrying mosquitoes were already resistant to the pyrethyroid insecticide used on bed nets and that disease rates were beginning to climb. “If you look at the insecticide-resistance profile of Africa, you’ll see that the heart of it is in West Africa,” Diabaté said when I spoke to him by phone last spring. “So when we were approached by Imperial College in 2012 about anti-malarial gene-drive mosquitoes, we thought, This is really something fantastic, really relevant for us, and we need to get involved in it.”
In collaboration with Target Malaria, Diabaté’s team conducted research and also began a gradual process of outreach and education. “We have tried to reach out to a lot of people,” Diabaté said. “From the grass-roots level, at the villages, to the top levels of the government — as well as journalists, other scientists, the religious community, the regional authorities.”
The person in charge of outreach in the villages, Dr. Léa Paré Toé, said the group began simply by taking stock. “We did a kind of baseline investigation,” she told me. “What’s the level of understanding of malaria? And we found that most people knew that malaria is transmitted by mosquitoes. But they also thought that rain can transmit malaria, or that it was caused by sweet food. So there was some confusion.”
Paré Toé and Diabaté began by explaining the biology of mosquitoes and walking residents through the lab’s routine activities: collecting mosquitoes to study breeding patterns or measuring the species’ range. “We also talked about the concept of research,” Paré Toé said, “because we needed to explain this idea to the community. It was new.”
The primary local language, Dioula, had no word for “gene” or “genetically modified,” so Paré Toé’s team also worked with linguists to develop a lexicon of terms. As Paré Toé described it to me, the group began by canvassing residents. “We would say, ‘Do you have a word in the local language that can explain these ideas?’ Then they come back with some words.”

Valentino Gantz, who invented the gene-drive technique with Ethan Bier, in his lab at the University of California, San Diego.
Craig Cutler for The New York Times
Afterward, the group hired a linguist, Dr. Daouda Traoré, to develop a glossary, which they checked against their own list and then field-tested. “For us, the most important thing was not to find a Dioulan term that was the equivalent of a particular phrase, like ‘genetic modification,’ but to find a way to explain what the concept actually means,” Traoré added. “The whole process took quite some time.”
At the same time, Target Malaria began working with the country’s regulatory agencies, including the National Biosafety Agency and the Ministry of the Environment, to create a staged approvals process. The first step, in 2016, was importing 5,000 mosquito eggs modified so that the males were sterile but did not carry a gene drive. (A release of sterile mosquitoes took place in July.) Assuming the current process continues, the first gene-drive mosquitoes would eventually be brought in from Italy — Burkina Faso does not have the lab facilities that would allow scientists to securely develop gene-drive mosquitoes — then further bred and tested in the lab to see, among other things, how effectively they can compete and mate with the endemic strains. (The main malaria-carrying mosquito in West Africa is Anopheles gambiae, but lab versions of the species are genetically different from wild Anopheles.)
Even so, Delphine Thizy, who acts as a liaison between Target Malaria and communities in Burkina Faso, estimates that it will be at least five years before the process to bring gene-drive mosquitoes to Africa can begin, and most likely a decade before anti-malarial gene-drive mosquitoes become available for any country to use (following approval by the World Health Organization). But she also cautions that the process could be much slower and that “if people reject it, it might just stop.”
While most African countries remain opposed to G.M.O. crops — in part because of their connection to multinational corporations — support for gene-drive technology to counter disease has so far remained high. (At the African Union summit meeting in 2016, the assembly established a panel to explore the use of emerging technologies, including the use of gene drives to eliminate malaria.) According to Hudu Mogtari, who works on regulatory support for emerging technologies for the pan-national African Union Development Agency, one important shift has been the collaboration between African and European scientists in the development of the technology, which has helped dispel accusations that Target Malaria is practicing “colonial medicine” and brainwashing villagers and African leaders — arguments that he says are primarily being pushed by Western anti-G.M.O. groups. “This is not something homegrown — that’s very obvious,” he told me. “These are professional activists.”
In August 2018, AUDA also started a program designed to facilitate discussion around an anti-malarial gene drive, with the goal of helping experts and ministers from different West African countries create shared guidelines for the technology. “We are dealing with a living modified organism that can cross borders,” Mogtari said dryly. “So we don’t have answers to some of the concerns that are being raised. But at least this platform would allow those concerns to be discussed.”
In the meantime, he added, the agency has started hosting information sessions for scientists from other fields, whom he describes as influential but often uninformed. “We have radio stations, TV stations, that will call up a scientist and say: ‘We have heard about this gene-drive technology. What do you say?’ But this may be a professor in a completely different field who has nothing to do with genetics or genome editing! And instead of being honest and saying, ‘I don’t know,’ they will talk. And whatever this person says, that will determine what people think. Because the lay public’s view is, once you say the person is a scientist, they must know everything.”
At the information sessions, Mogtari recalled, questions have ranged from comparatively informed (Would eliminating Anopheles affect the food chain?) to more absurd (Would modified mosquitoes suddenly become able to transmit H.I.V.?). When I mentioned this to Dr. Diabaté, he laughed. “People don’t have a really good understanding of the biology of mosquitoes, and the malaria parasite, and how the interaction between these two allows a mosquito to transmit a certain disease,” he said. “But these are concerns that people have very often, so you have to address it.”
Mogtari agreed. “Usually when people attend the meetings for the first time, you can tell from their comments,” he told me. “It’s things they have picked up from the media. About how mosquitoes will grow to the size of helicopters. Or how you can have something that’s half human, half mosquito. And it’s good, because as we hold our meetings, you really see the change. People who are vehemently against this, when the facts are given to them, they change their minds completely. And, you know, it’s gratifying, when you go through that process. But there also are a lot more people out there. Not everyone can come to these meetings.”
In his book “The Wizard and the Prophet,” the journalist Charles C. Mann writes that there are two kinds of people: wizards, who see science and technology as our best hope for human survival, and prophets, who believe that the human race will survive only if we can limit our growth and live simply, reversing the changes wrought by modern agriculture, development and consumption.
In practice, most of us are a bit of both. We want cars and airplanes, laptops and electric lights, cheap food and medications that work. Our lives, we understand, are far better than they would have been 200 years ago, let alone 400. Despite all this, it’s hard not to worry about the cost. Deforestation, climate change, entire species gone from the earth — it reads like a catalog of our sins, the price of our progress. More than that, we suspect that it will be our undoing.
This seems especially true at a time when a single rogue scientist has the power to upend years of careful constraint. While the Convention on Biological Diversity was underway, He Jiankui, a researcher at the Southern University of Science and Technology in China, announced that nine months earlier, he had used the gene-editing technique Crispr to alter embryos, which he then implanted in the womb of a woman. That woman gave birth to twin girls, creating the world’s first genetically edited babies. The news caused an uproar, in part because He created the embryos despite an agreement among researchers that germ-line editing in human embryos was still too risky to be used outside the lab. The director of the National Institutes of Health, Dr. Francis S. Collins, issued a scathing statement citing the “deeply disturbing willingness by Dr. He and his team to flout international ethical norms.” Even though He was later sentenced to three years in prison, the genie was out of the bottle.
In practice, a working gene drive is harder to make, and to deploy, than a single edited embryo. But the risk is clear: There are limits to the effectiveness of scientific self-policing. As Jim Thomas of ETC Group says: “So far, all the proposals around gene drives are things like voluntary ethics codes and agreements between funders. They’re not binding in any way, so to what extent they can be enforced and who would be liable in the event of problem — there’s none of that.”
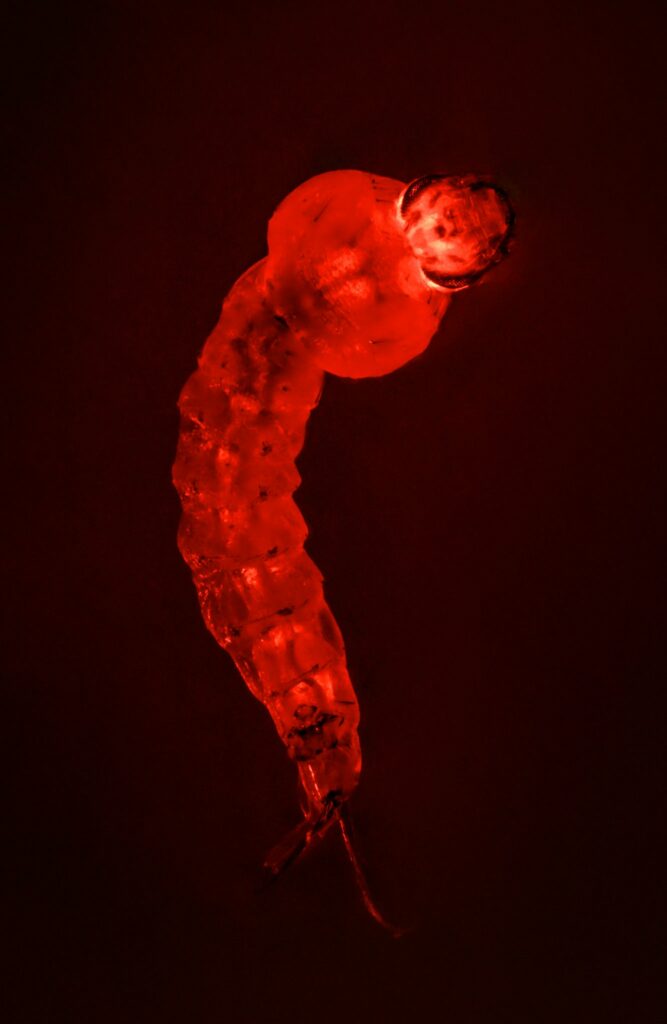
An Anopheles stephensi larva with fluorescence from a gene drive, in the lab of the biologist Anthony James at the University of California Irvine. Craig Cutler for The New York Times
Since the United Nations convention, Kofler and others working on gene-drive regulation have emphasized the need for community involvement and “informed consent” — both as a moral good (people deserve to have a say in decisions that will affect them) and for practical reasons (people are more likely to reject a technology if it feels imposed from the outside). Both sides also support the creation of a neutral global regulatory system: something trusted, transparent and enforceable, with punitive liabilities. But it would most likely be hard to agree on what a “neutral” system should look like. As Thomas sees it, the global institutions that are already engaged in the discussion around gene drives — the United Nations, the World Health Organization — are unfairly influenced by scientists and gene-drive proponents. “We need to move away from an expert-o-cratic process,” he told me.
But it’s hard not to feel nervous about a regulatory system in which lay and expert opinion is given equal weight. Do we really want the process of scientific research and technology to become democratic — one in which fundamental decisions about public health, like vaccines and vector-control measures, are put up for a vote? It is difficult for most of us to sift through a welter of complex facts and claims, however much we may push for “informed consent.” “What does ‘public engagement’ really mean in this context?” says Elizabeth Heitman, an ethicist at the University of Texas Southwestern who has studied the public reaction to emerging technologies. “It means talking about a developing science with a lot of uncertainties and a really steep learning curve.”
Like most of the researchers I spoke with, James regards the uncertainties around gene drives more as questions to be answered than as perilous or unknowable unknowns. “When I’ve talked with people about these things, they usually say, ‘But you don’t know what’s going to happen!’ ” James told me. “But that’s why you do experiments. That’s why you do them for years.”
If this attitude sounds slightly wishful — no lab experiment can capture every possible interaction or effect of a gene drive in the wild — it also seems to reflect something more fundamental: the gap between how scientists and laypeople think about efficacy and risk. James told me that he grew up as one of 10 children in a family that was often financially strapped but also intellectually rigorous. His mother, who grew up in Minnesota, studied library science. His father was a mathematician and aerospace engineer for North American Rockwell, where, among other things, he helped design rocket engines for the Apollo spacecraft. As an interracial family in the 1950s — James’s father was black, his mother white — “there wasn’t a lot of bowing to institutional doctrines,” he told me. Instead, the children absorbed a mix of pragmatic calculation and logical reasoning. “With that many kids, you had to be pretty empirical in your decision-making.”
In college, James decided to major in biology and began working in a lab that was trying to find the genetic roots of development by studying abnormalities in fruit flies — an early version of Gantz’s research. At the time, James recalled, he had a roommate whose huge, shaggy dog became infested with fleas. When James complained, the roommate suggested making a collar of eucalyptus buds, which he argued would function as a natural repellent. James had a better idea. “I thought, I can fix this in half an hour,” he recalled. The lab’s stores included a bottle of the pesticide malathion, and that evening he brought a small amount of the powder back to the room “and sort of sprinkled it around.” By the next morning, the fleas were gone.
When James told me this story, I wondered if he understood how disturbingly incautious it made him seem. But as he went on, I realized this might not be a fair reading. As part of the same conversation, James mentioned that his great-grandmother, who was Creole, fled New Orleans in the late 1800s in order to escape a yellow-fever epidemic — one that would later kill more than one-tenth of the entire population of Memphis.
At the time, yellow fever was thought to be an infection that was carried on dirty clothes and spread through physical contact. A few years later, a military physician, Maj. Walter Reed, and a Cuban physician and scientist, Carlos Finlay, proved that Aedes aegypti mosquitoes were causing the outbreaks. The result was a sweeping eradication campaign. Lakes and swamps were drained. Those that remained were coated with oil or pesticides that killed mosquito larvae. The project saved thousands of lives and transformed the United States. Would doing the same to the forests and savannas of Africa be more or less invasive than using a gene drive to eliminate Anopheles mosquitoes?
With its ability to create powerful changes invisibly, genetic engineering can feel eerie to even the most rational of us. Brain-imaging studies of people who strongly oppose genetically modified foods show that simply imagining, say, eating a G.M.O. tomato will cause some people to have a powerful disgust response, as if they were being asked to eat worms. When I mentioned this to James, he seemed unsurprised. “People are sort of weird about genes,” he said with a shrug. “There’s a visceral fear. And it’s hard to talk people out of those sorts of fears.”
At my request, James led me down to the insectary where his lab at U.C. Irvine breeds genetically engineered mosquitoes to study, for example, whether they can be made malaria-resistant. Because James didn’t work with the malaria parasite itself — he outsourced that part of the research to a containment facility in San Diego — the insectary itself was comparatively low-security, but it still had multiple doors, rooms designed to have negative pressure and an industrial-strength plastic curtain of the kind you see on loading docks.
For all that, the facility felt unexpectedly homely: just four small rooms with wire shelves and containers for the different mosquito crosses — in this case, movie-theater popcorn tubs sealed at the top, then retrofitted using a piece of mosquito net stapled over a hole in the bucket’s side. (James’s lab goes through a lot of containers, and these were the cheapest his lab tech could find.) The effect was incongruously cheery, like a grade-school science project in some wacky high-tech future. On each tub, the netting was flanked by the word “Popcorn!” on a bright yellow background.
I asked James if I could see a mosquito carrying a gene drive, and he pointed to one of the buckets. But when I peered in, I couldn’t help feeling disappointed. Although I knew that a gene drive wouldn’t be visible — it was just a short stretch of DNA, after all — the TV-watching part of me had still expected something. Instead, I saw what appeared to be an ordinary mosquito — which, after a moment, felt almost more alarming.
Among malaria researchers, the main worry isn’t that gene drives will be too powerful but that they won’t be powerful enough. For one thing, it’s unclear whether a gene drive — which can rapidly change the offspring of hundreds of mosquitoes held captive in a cage — will do the same in the real world. “In the wild, mosquitoes are very dispersed,” James told me. “And it’s not clear how much they’re interacting with each other — there are mountains, rivers. It’s so stochastic. If the one male in that area dies, that’s it for that area.”
‘How do you regulate a technology that’s undetectable, self-propagating and can fly?’
In the wild, even a small genetic change almost always incurs what scientists call a fitness cost: Either an engineered insect won’t be as hardy as its wild peers or it won’t be an attractive mate. (Simply changing the fur color of a fruit fly from brown to yellow, as Gantz did, for instance, reduces its chance of mating by 99 percent.) More radical changes, like creating a mosquito that produces only male offspring, are likely to face even more resistance. Nature is good at circumventing anything that thwarts procreation.
Delphine Thizy of Target Malaria told me that because of these factors, the foundation didn’t expect gene drives to actually eliminate malaria. “The goal is really just to deplete the mosquitoes from an area enough that the parasite-insect-human cycle collapses,” she added. “If you look at all the obstacles — the physical obstacles, like geography, as well as the evolutionary pressures — it’s more likely that even a really well-engineered gene drive won’t spread as well as we’d think.”
Current research suggests that the spread of gene drives is likely to vary from species to species, with some propagating slowly, if at all, and others more rapidly or widely. Research also suggests that gene drives stay confined to a single species rather than spreading into a related one through interbreeding. But it’s not clear whether that will be true in all species or under all conditions. (Researchers are also working on a variety of containment strategies, including drives that stop working after a few generations.) And it’s very hard to assess what the environmental impact of removing a species, or even altering one, might be. While ecosystems tend to be resilient — plenty of species have gone extinct already, and it hasn’t led to a systemic collapse — they’re also complicated and difficult to model. The only way to conclusively determine what happens when a species changes or vanishes may be to try it and see.
Ethan Bier, who has become deeply involved with the technology since his and Valentino Gantz’s breakthrough, emphasized that the many potential applications are likely to have extremely different benefits and risks. Malaria, he noted, is one of the strongest cases. Studies show that reducing or even eliminating the Anopheles mosquito is unlikely to have a significant environmental effect (few birds or animals rely on it as a food source), and as it is one of 3,500 mosquito species on the planet, its disappearance wouldn’t appreciably dent the insect’s overall diversity. And given that malaria kills hundreds of thousands of people a year, the argument for not using a gene drive would have to be unusually strong. Bier recalled one early conversation in which Gantz asked: “Imagine you could genetically engineer a mosquito that would prevent you from getting cancer. Would people still object to it?”
In conservation and agriculture, gene drives could also have a profound effect, with the potential both to save endangered species and to reduce the amount of pesticides currently in use. But these, too, carry risks. New Zealand has discussed using a gene drive to eradicate the Australian brushtail possum, which preys on the nests of native birds and is currently controlled with poison traps. But should a few dozen Australian possums with an all-male gene drive be carried from New Zealand back to Australia, they could devastate the native possum population. Agricultural uses are even more fraught. If a corporation wants to use a gene drive to “cancel out” the herbicide resistance that some weeds have now developed, would that really benefit the planet — or just the corporation that can now sell more of the herbicide that caused the problem in the first place?
In theory, figuring out how to answer these questions should be the province of the world’s regulatory agencies, and most scientists agree that gene drives will need to be evaluated on a case-by-case basis, akin to how the Food and Drug Administration evaluates the safety of a new treatment or pharmaceutical. But regulating a technology that doesn’t stop at the border of a country or a state is a new problem. Unlike a chemical pesticide, gene drives are inherently mobile — able to cross borders or potentially even oceans. And while some species, like the malaria-carrying Anopheles gambiae mosquito, exist only in sub-Saharan Africa, others, like the Norway rat, are virtually everywhere. As Kuiken put it: “How do you regulate a technology that’s undetectable, self-propagating and can fly? If one community doesn’t want it, does that mean that the other four or five communities around it aren’t allowed to move forward? How do you set up an international governance regime that enables you to make those kinds of decisions? So far, I haven’t seen any proposals that get us there.”
The United Nations and the International Union for Conservation of Nature have created working groups to study the problem and begin to hash out best practices around gene-drive use, though these may be difficult to enforce. A handful of countries have been more rigorous. In June 2018, the National Institute for Public Health and the Environment in the Netherlands passed legislation that included a detailed evaluation process for any gene drive to be used outside the lab.
So far, the United States has yet to take similar steps. Zach Adelman, an entomologist who works on gene drives at Texas A&M, told me that until recently federal agencies “put their head in the sand” around the question of gene drives. “We’ve been trying to get the attention of regulators to say: ‘Hey, we’re developing this technology. Can we start to talk about how it might be regulated, and what we need to do, what we need to change?’ ” Adelman told me. “And that got no traction for a long time.”
In the past year, the agencies finally began to act. Adelman says that the Department of Agriculture is now working to develop a risk-assessment process for agricultural gene drives, and the Environmental Protection Agency and the F.D.A. are also reportedly taking an interest. “We’ve lost a few years, but now it’s definitely on their radar,” Adelman says. Still, the agencies’ guidelines remain vague. “For now, people doing the work have been policing themselves,” Adelman told me. “Which will work — right up until it doesn’t.”
Is it O.K. to engineer the environment to fight climate change?
As I drove back from James’s lab, the sky was hazy. It was summer, and fires were burning in the mountains behind Los Angeles, filling the skies with smoke. In that moment, it seemed as if the prophets were right, and our relentless progression had irrevocably tipped the balance from innovation and growth to disaster and decline.
Between the artificial-intelligence apocalypse and the designer-babies apocalypse and the actual apocalypse (melting glaciers, plastic in the oceans), it is often hard to escape the feeling that we are, increasingly, using technology to fix problems that technology itself has created. As bees die off because of pesticides, there’s talk of using tiny drones to pollinate crops. Global warming is already generating plans for geoengineering: seeding the stratosphere with reflective particles to limit the sun or filling the ocean with crushed limestone to reduce its acidity. Such practices can feel like a high-tech version of introducing rabbits to keep down the weeds, and then foxes to keep down the rabbits. It’s tempting to say we should just stop meddling. Nature, after all, is supposed to be natural. Should it become possible to alter wild species en masse, at a genetic level, how will that affect our idea — or, perhaps more accurate, our fantasy — of an unspoiled world? And what will it mean for our relationship to the other creatures on the planet?
“This notion of permanently altering the genetics of an entire species — it goes against everything I was trained to think,” says Kuiken, who served on the United Nations’ technical experts committee for gene drives. “What’s hard to accept is that, at this point, it might end up being our best option. There’s this kind of fantasy that we can go back, that we can restore some lost Eden. But the reality is that we aren’t making those choices.”
And even if we could, would it make sense to do so? After all, the rise in antibiotic resistance doesn’t mean that we shouldn’t have invented antibiotics at all. Yet innovations inevitably change how we behave, and those changes have consequences. As Kuiken put it: “You kind of have to accept that we’ve failed, societally. That we’re going to continue to drive, to fly, to throw away plastic, to tear down the rainforest. And if we aren’t going to solve the problems we’ve created by regulating ourselves, it means that we’re probably going to have to use technology — whether that’s to save species, or human lives, or to make sure that certain plants or coral reefs survive climate change.”
Kuiken paused: “That’s part of why all this is so hard. It’s not just a question of whether or not we should use gene drives. It’s about coming to grips with our failures.”
______________________________________________
Related Coverage:
- From Gene Editing to A.I., How Will Technology Transform Humanity? 16, 2018
- The Crispr Quandary 9, 2015
- Is It O.K. to Tinker With the Environment to Fight Climate Change? April 18, 2017
- Is Ancient DNA Research Revealing New Truths — or Falling Into Old Traps? 17, 2019
Jennifer Kahn is a contributing writer for the magazine and a lecturer at the U.C. Berkeley Graduate School of Journalism.
A version of this article appears in print on Jan. 12, 2020, Page 26 of the Sunday Magazine with the headline: Unnatural Selection.
Tags: Ethics, GMO, Genetic engineering, Genetic manipulation, Nature's Rights, Research, Science, Science and Medicine
DISCLAIMER: The statements, views and opinions expressed in pieces republished here are solely those of the authors and do not necessarily represent those of TMS. In accordance with title 17 U.S.C. section 107, this material is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. TMS has no affiliation whatsoever with the originator of this article nor is TMS endorsed or sponsored by the originator. “GO TO ORIGINAL” links are provided as a convenience to our readers and allow for verification of authenticity. However, as originating pages are often updated by their originating host sites, the versions posted may not match the versions our readers view when clicking the “GO TO ORIGINAL” links. This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond ‘fair use’, you must obtain permission from the copyright owner.