Contradictions Emerge Amid the Race to Develop a COVID-19 Vaccine
COVID19 - CORONAVIRUS, 7 Dec 2020
Benjamin Mateus | WSWS - TRANSCEND Media Service
3 Dec 2020 – A full year has passed since the SARS-CoV-2 virus first emerged in China’s Hubei province—sometime between mid-October and mid-November of 2019, according to a recent collaborative study from the University of California San Diego and the University of Arizona posted to a preprint server this week.
While the public health response to the spread of the coronavirus has shown the complete incapacity of the capitalist system to safeguard the lives and health of the people, the effort to develop a vaccine—where the drive for profit was focused—has borne fruit relatively quickly.
In part this is due to the unprecedented speed and energy that characterized the initial work of scientists to discover the genetic code of the novel virus, the necessary precondition for developing a vaccine using a biochemical process involving messenger RNA (mRNA).
By the end of December, Wuhan’s health systems had begun to identify a series of concerning pneumonia cases of an unknown cause whose clinical picture resembled viral pneumonia. On December 26, 2019, an elderly couple with high fevers and cough was admitted to a local Wuhan hospital. A chest CT scan demonstrated findings completely different from other viral types of pneumonia. Their asymptomatic son had similar findings on his chest CT. The common viral pathogens, such as influenza and syncytial virus, were ruled out on tests.
With clinical and radiological information on other recently admitted patients, Dr. Zhang Jixian, director of the department of Respiratory and Critical Care Medicine at Hubei Hospital of Integrated Traditional Chinese and Western Medicine, suspected they were confronting an as of yet unidentified pathogen with epidemic potential. Because of its connection with many that were infected, the seafood market was shuttered for cleaning and disinfection on January 1.
On January 3, Professor Zhang Yongzhen of Fudan University at the Shanghai Public Health Clinical Centre received test tubes of swabs taken from some of the patients admitted in Wuhan. In less than 48 hours, he had mapped the first complete genome of the virus, now better known as SARS-CoV-2. Over the next several days, they had confirmed that the recent respiratory illnesses were caused by a novel coronavirus, sending shock waves within the small niche in the scientific community and public health departments.
Over the potential for a public health crisis from a SARS-like novel coronavirus, on January 11, Professor Yongzhen instructed his associate, Professor Edward Holmes of University of Sydney, to upload the sequence to the website Virological.org which allowed the outside world access to the complete genetic code. Interestingly, in response to his critics about a cover-up, as reported in Time, he explained that they had uploaded the genome sequence to the US National Center for Biotechnology Information on January 5 after mapping the coronavirus.
The race to develop a vaccine took on an immediate urgency over the next few weeks as news of Wuhan’s outbreak began to spread. The earliest report was provided by Dr. Anthony Fauci who had told CNN on January 20 that the National Institute of Health (NIH) was in the process of taking the first steps towards the development of a vaccine in collaboration with then relatively unknown biotech company named Moderna. Soon many large biotechnology companies and pharmaceuticals worldwide had turned their attention to making a vaccine against the coronavirus. There are, as of this writing, 13 vaccines in phase three, 17 in phase two, and 40 in phase one human trials. Numerous others remain in the preclinical phases.
Moderna, Pfizer and mRNA vaccines
Stéphane Bancel, CEO of Moderna, an American biotechnology company based in Cambridge, Massachusetts, recounts to the New York Times that he was on a business trip in Switzerland when he heard of China’s epidemic. He turned to his connections at the National Institutes of Health (NIH), with whom his company had been working for years to develop a novel approach to vaccine designs.
The centuries-long history of human experiences with pandemics has frequently been catastrophic. Out of the study of many tragedies and the comprehension of the natural world, which included understanding the microscopic nature of these pathogens and the immune system’s response, has come the discovery of many lifesaving vaccines.
Still, processes that require the injection of weakened or inactivated viruses, as with smallpox, tend to be laborious, taking several years of investigation and research to realize a potential candidate. In the face of a pandemic, however, time-sensitive therapeutics becomes essential, and non-pharmaceutical interventions such as public health measures remain the mainstay in responding to these health crises.
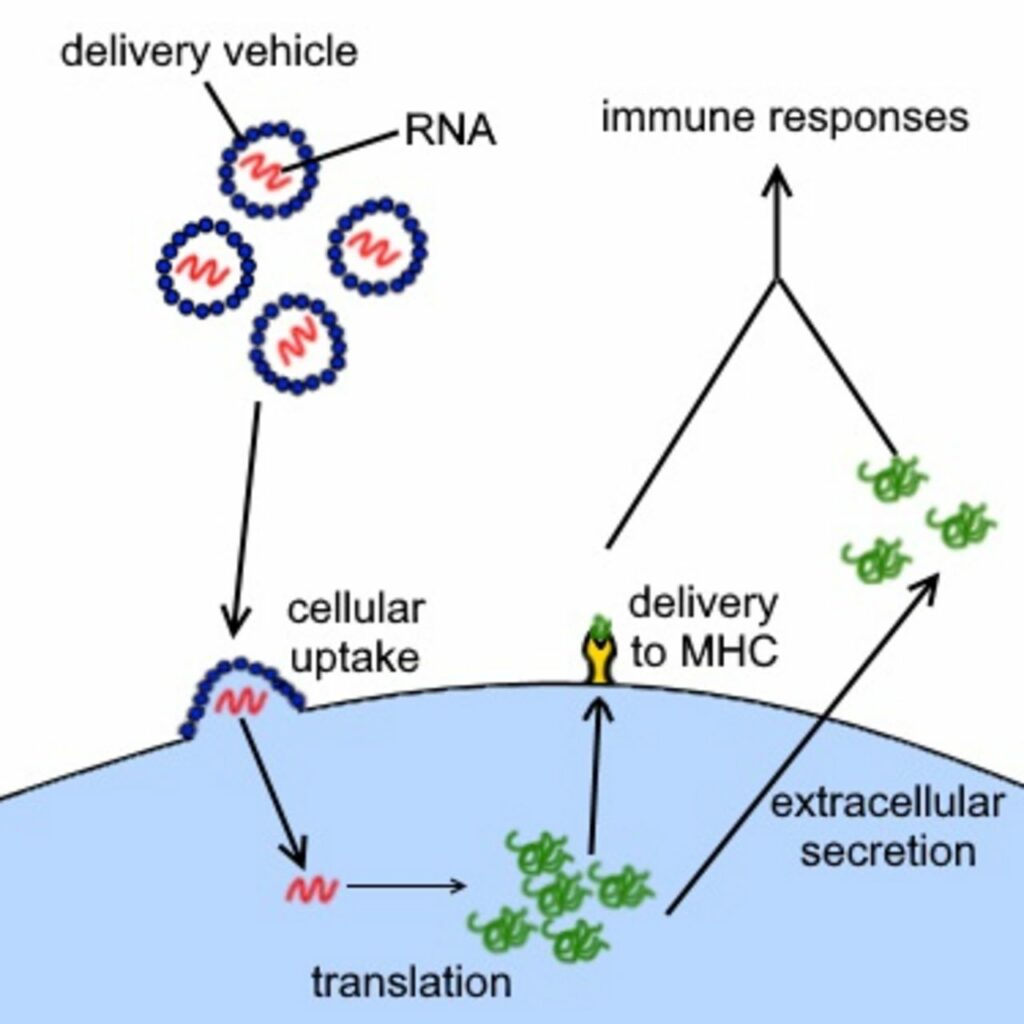
But more recently, with advances in genetics and bioengineering, the approach to vaccine development has also undergone a paradigmatic shift that can possibly provide such treatments in real time. As described in the New York Times, “Moderna and other companies created platforms that work like the operating system on a computer, allowing researchers to quickly insert a new genetic code from a virus—like adding an app—and create a new vaccine.” This means that by providing a person with the appropriately constructed genetic material, their cells can take these “synthetic genetic codes” and translate them into harmless mimic viral proteins that will stimulate their immune system and generate antibodies to protect them against the real pathogen.
After zeroing in on the coronavirus’s spike protein for their vaccine target, Moderna had only to input the necessary genetic sequences into their computer programs. Within two days, it had designed a messenger RNA (mRNA) vaccine candidate. In 25 days, the prototype of the vaccine had been manufactured, and in just 42 days, on February 24, it had been shipped for testing.
Up to then, Moderna had never produced an approved drug or vaccine. Its finances relied solely on the potential for its genetic platform to create these therapeutics. Previous efforts to test new vaccines when the SARS, MERS, and Zika outbreaks occurred were thwarted as the threat receded too quickly for large human clinical trials to be conducted. The scale and duration of the COVID-19 pandemic, raging across densely populated regions of the world, were critical in proving that these concepts could be applied in practical terms. But, given its limited resources, Moderna’s successes over the intervening months were highly dependent on the critical collaboration with NIH investigators and funding support from the Coalition for Epidemic Preparedness Innovations.
By comparison, the behemoth drug manufacturer, Pfizer, was a late starter in the vaccine race. On March 1, they were approached by their collaborative partners Dr. Ugur Sahin and Dr. Özlem Türeci. This couple owns the German biotechnology company BioNTech, which manufactures immunotherapies and messenger RNA therapeutics as means of individualized cancer treatments. However, Dr. Sahin also recognized its immense potential in vaccinating against epidemic potential pathogens. At an infectious disease conference in Berlin two years prior, he had even predicted that messenger RNA technology could rapidly develop new vaccines in the event a global pandemic was to strike.
Around the same time that Stéphane Bancel had recognized the potential opportunity the Wuhan outbreak offered, Dr. Sahin had become convinced the novel coronavirus epidemic exploding across Hubei province would materialize into a global health crisis. He told the Times, “There are not too many companies on the planet which have the capacity and the competence to do it so fast as we can do it. So, it felt not like an opportunity, but a duty to do it, because I realized we could be among the first coming up with a vaccine.”
The messenger RNA breakthrough
Both Moderna and Pfizer have banked on a genetic technology that uses synthetic messenger RNA which turns a person’s cell into a vaccine manufacturing machine producing mimic proteins. The immune system can recognize and form antibodies to protect itself against future exposure to the actual pathogen.
Briefly, one of the DNA’s primary functions, which resides in a cell’s nucleus, is to produce proteins. The appropriate portion of the DNA is unwound, and a single strand of messenger RNA is transcribed. It undergoes further processing into its mature form and is then transported to the cell’s cytoplasm, waiting to be read. Ribosomes, proteins that can decode the “message” contained in the mRNA, utilizing amino acids carried by transfer RNA (tRNA), then set to build the protein according to specification, after which they are presented to the immune system. Because the spike proteins are only one small component of the entire virus, these mimics are harmless.
Previous vaccines have used attenuated or devitalized viruses or specific peptides and proteins derived from these pathogens to create vaccines. In contrast, mRNA vaccines use a person’s cells as manufacturing sites. This has practical importance in reducing the scale and time for developing the vaccines.
The potential for mRNA therapeutics goes beyond that of vaccines. For decades, scientists have pondered the potential role of synthetic mRNA technology in treating various diseases such as mending damaged hearts, defective enzymes that cause rare diseases, or cancers. It was back in 1990, for the first time, researchers at the University of Wisconsin working with mice injected RNA and DNA “expression vectors” into skeletal muscle that resulted in new protein expressions.
A Hungarian biochemist named Katalin Karikó decided to push the envelope in this field. Synthetic RNA is readily vulnerable to a human’s natural defenses and can elicit a massive immune response making the therapy a health hazard. After a decade’s work with multiple trials and errors, she and her collaborator at Penn, Drew Weissman, an immunologist, recognized that by modifying the mRNA’s building blocks, they could deliver it into cells without the immune system becoming alerted to these intruders and attacking them.
Though their studies went unrecognized back in 2005 by the scientific community at large, they were of immense significance that would provide a practical solution to discovering novel therapies for diseases that hitherto had no treatments. However, they did catch the attention of a select few scientists who would go on to found the biotechnology firms Moderna and BioNTech.
A meeting in 2010 between Derrick Rossi, a stem cell biologist at Harvard University, who pitched the idea behind an mRNA technological startup to Robert Langer, a well-established biomedical engineer from MIT turned entrepreneur, and Noubar Afeya, a venture capitalist, led in a matter of months to the formation of the firm Moderna. Stéphane Bancel was brought on board as CEO in 2011 to help the company build its investors’ and financiers’ ranks. Rossi left the company in 2014 over a bitter dispute over who conceptualized the far-reaching implications of this new technology.
In a similar vein, the husband-and-wife team of Dr. Sahin and Dr. Türeci were enticed by the concept of personalized immunotherapies that could teach a person’s immune cell to fight cancer cells. With financial support from German sources, BioNTech was formed with its headquarters in Cambridge, Massachusetts, just a few miles from Moderna.
Though both companies are using an mRNA vaccine, the vaccines’ chemical structures, how they are produced, and how they are delivered into cells are different. Both also require stringent temperature requirements due to their sensitivity to degradation. Pfizer’s needs to be stored at minus 94 degrees Fahrenheit, which makes the logistics of transporting the vaccine, storing, and administering a daunting task.
This gives an edge to Moderna’s vaccine, which requires long-term storage at only a modest minus 4 degrees Fahrenheit and can remain stable for a month at 36 degrees to 46 degrees Fahrenheit. The Moderna vaccine’s stability is attributed to its special membrane made of lipid nanoparticles (tiny oily spheres) surrounding and protecting the mRNA from degradation at higher temperatures. Both vaccines require two injections to complete the series, 21 days apart for Pfizer and 28 days for Moderna.
A week after Pfizer emerged as the first vaccine candidate to show a dramatic 90 percent efficacy against the virus in randomized phase three clinical trials, on November 16, Moderna’s much-awaited announcement on results from their interim analysis corroborated the mRNA technology as a powerful tool. Moderna initially bested Pfizer with a show of 94.5 percent until revised and updated results show the two vaccines virtually equal in efficacy.
Moderna disclosed that their data included people in high-risk groups, such as those over 65. There were 90 cases of COVID-19 in the unvaccinated group, with eleven severe cases in their interim analysis. In the five infections among those that received the vaccine, no severe symptoms developed. Pfizer’s recent updated analysis revealed that out of 170 COVID-19 infections, only eight had taken the vaccine. Additionally, in a review of 8,000 subjects, no serious safety issues were encountered; 3.8 percent had reported severe fatigue and 2 percent headaches. The transient adverse effects for Moderna’s vaccine include the report of 9.7 percent fatigue, 8.9 percent muscle pain, 5.2 percent joint pain, and 4.5 percent headaches.
Arnold Monto, an epidemiologist at the University of Michigan School of Public Health, explained, “this is higher reactogenicity than is ordinarily seen with most flu vaccines, even the high dose ones.” Vaccine experts are concerned this will have considerable impact on how these therapeutics are received by the population.
On November 20, Pfizer CEO Albert Bourla announced that their vaccine had sufficient safety data and had filed an emergency use authorization (EUA) with the US Food and Drug Administration. Moderna filed for its own EUA ten days later.
From development to mass vaccination
The FDA’s Vaccines and Related Biological Products Advisory Committee will meet from December 8 through 10 to review the Pfizer application, and then a week later for Moderna. Decisions may be forthcoming immediately. By all accounts, both vaccines will most likely be granted approval as they both use an mRNA vector that appears to have similar safety and efficacy results.
Then follows a review by the CDC Advisory Committee on Immunization Practices that will issue guidance on who can receive the vaccine and which groups will be prioritized. The consensus among public health experts is to allocate an initial lot of vaccines to immunize health care workers. Other groups to be given priority include the elderly and essential workers, such as police officers, who are classified as first responders.
However, the task of manufacturing, delivering, and immunizing the entire planet with the same speed with which these lifesaving treatments have been developed is, literally, unprecedented. Significantly, the Information Technology infrastructure is lacking to track who has received which vaccine and how side effects and reactions are reported. Science has been able to penetrate into nature’s most compelling secrets, but the capitalist mode of production—along with the outmoded system of nation-states—constitute the main barriers to saving millions of lives.
The executives and shareholders of the Moderna, Pfizer, BioNTech and other vaccine manufacturers will undoubtedly grow rich—and from the standpoint of the profit system, that is the only concern—but a return to normal conditions of life for the great mass of humanity remains in considerable doubt.
On May 15, the Trump administration launched Operation Warp Speed as a public-private partnership to “facilitate and accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics.” In effect, drug companies are using public funds to ensure the profitability of their development and manufacturing, the US military has been assigned the task of wholesale distribution, from the manufacturers to the various states, and state governments will oversee the retail distribution and mass vaccination, including deciding who gets the vaccine and when. To call this arrangement a Rube Goldberg device would be to give it too much credit. It is a disaster waiting to happen.
And then there is the critical question of global distribution, particularly in poor nations which lack the health care infrastructure even to distribute childhood vaccinations that are commonplace in the advanced countries, let alone deliver two shots, weeks apart, to every single person in the country.
In a press release published on September 17, OXFAM International reported that wealthy nations representing just 13 percent of the world’s population had monopolized more than 50 percent of all future doses of COVID-19 vaccines. They warned that even if the five leading vaccines (there are presently 12 in phase three) prove successful, 61 percent of the world’s population will not see a vaccine until 2022. Currently, the WHO COVID vaccine global initiative is struggling to raise the necessary funds to distribute these vaccines to the worldwide population equitably.
Tags: Airborne contagion, COVID-19, China, Community, Compassion, Coronavirus, Cuba, Economy, Empathy, Environment, Health, Lockdown, Orthomolecular Medicine, PCR Tests, Pandemic, Public Health, Research, Science, Science and Medicine, Swiss Policy Research, United Nations, WHO
DISCLAIMER: The statements, views and opinions expressed in pieces republished here are solely those of the authors and do not necessarily represent those of TMS. In accordance with title 17 U.S.C. section 107, this material is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. TMS has no affiliation whatsoever with the originator of this article nor is TMS endorsed or sponsored by the originator. “GO TO ORIGINAL” links are provided as a convenience to our readers and allow for verification of authenticity. However, as originating pages are often updated by their originating host sites, the versions posted may not match the versions our readers view when clicking the “GO TO ORIGINAL” links. This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond ‘fair use’, you must obtain permission from the copyright owner.
Read more
Click here to go to the current weekly digest or pick another article:
COVID19 - CORONAVIRUS: