Reducing Transmission of SARS-CoV-2
COVID19 - CORONAVIRUS, 1 Jun 2020
K. A. Prather, et al. | Science Magazine - TRANSCEND Media Service
Masks and testing are necessary to combat asymptomatic spread in aerosols and droplets.
27 May 2020 – Respiratory infections occur through the transmission of virus-containing droplets (>5 to 10 μm) and aerosols (≤5 μm) exhaled from infected individuals during breathing, speaking, coughing, and sneezing. Traditional respiratory disease control measures are designed to reduce transmission by droplets produced in the sneezes and coughs of infected individuals. However, a large proportion of the spread of coronavirus disease 2019 (COVID-19) appears to be occurring through airborne transmission of aerosols produced by asymptomatic individuals during breathing and speaking (1–3). Aerosols can accumulate, remain infectious in indoor air for hours, and be easily inhaled deep into the lungs. For society to resume, measures designed to reduce aerosol transmission must be implemented, including universal masking and regular, widespread testing to identify and isolate infected asymptomatic individuals.
Humans produce respiratory droplets ranging from 0.1 to 1000 μm. A competition between droplet size, inertia, gravity, and evaporation determines how far emitted drop-lets and aerosols will travel in air (4, 5). Respiratory droplets will undergo gravitational settling faster than they evaporate, contaminating surfaces and leading to contact trans-mission. Smaller aerosols (≤5 μm) will evaporate faster than they can settle, are buoyant, and thus can be affected by air currents, which can transport them over longer distances. Thus, there are two major respiratory virus transmission pathways: contact (direct or indirect between people and with contaminated surfaces) and airborne inhalation.
In addition to contributing to the extent of dispersal and mode of transmission, respiratory droplet size has been shown to affect the severity of disease. For example, influenza virus is more commonly contained in aerosols with sizes below 1 μm (submicron), which lead to more severe infection (4). In the case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is possible that submicron virus-containing aerosols are being transferred deep into the alveolar region of the lungs, where immune responses seem to be temporarily bypassed. SARS-CoV-2 has been shown to replicate three times faster than SARS-CoV-1 and thus can rapidly spread to the pharynx from which it can be shed before the innate immune response becomes activated and produces symptoms (6). By the time symptoms occur, the patient has transmitted the virus without knowing. Identifying infected individuals to curb SARS-CoV-2 transmission is more challenging compared to SARS and other respiratory viruses because infected individuals can be highly contagious for several days, peaking on or before symptoms occur (2, 7). These “silent shedders” could be critical drivers of the enhanced spread of SARS-CoV-2. In Wuhan, China, it has been estimated that undiagnosed cases of COVID-19 infection, who were presumably asymptomatic, were responsible for up to 79% of viral infections (3). There-fore, regular, widespread testing is essential to identify and isolate infected asymptomatic individuals.
Airborne transmission was determined to play a role during the SARS outbreak in 2003 (1, 4). However, many countries have not yet acknowledged airborne transmission as a possible pathway for SARS-CoV-2 (1). Recent studies have shown that in addition to droplets, SARS-CoV-2 may also be transmitted through aerosols. A study in hospitals in Wuhan, China, found SARS-CoV-2 in aerosols further than 6 ft from patients with higher concentrations detected in more crowded areas (8). Estimates using an average sputum viral load for SARS-CoV-2 indicate that 1 min of loud speaking could generate >1000 virion-containing aerosols (9). Assuming viral titers for infected super-emitters (with 100-fold higher viral load than average) yields an increase to more than 100,000 virions in emitted droplets per minute of speaking.
The World Health Organization (WHO) recommendations for social distancing of 6 ft and hand washing to reduce the spread of SARS-CoV-2 are based on studies of respiratory droplets carried out in the 1930s. These studies showed that large, ~100 μm droplets produced in coughs and sneezes quickly underwent gravitational settling (1). However, when these studies were conducted, the technology did not exist for detecting submicron aerosols. As a comparison, calculations predict that in still air, a 100-μm droplet will settle to the ground from 8 ft in 4.6 s whereas a 1-μm aerosol particle will take 12.4 hours (4). Measurements now show that intense coughs and sneezes that propel larg-er droplets more than 20 ft can also create thousands of aerosols that can travel even further (1). Increasing evidence for SARS-CoV-2 suggests the 6 ft WHO recommendation is likely not enough under many indoor conditions where aerosols can remain airborne for hours, accumulate over time, and follow air flows over distances further than 6 ft (5, 10).
In outdoor environments, numerous factors will deter-mine the concentrations and distance traveled, and whether respiratory viruses remain infectious in aerosols. Breezes and winds often occur and can transport infectious droplets and aerosols long distances. Asymptomatic individuals who are speaking while exercising can release infectious aerosols that can be picked up by air streams (10). Viral concentrations will be more rapidly diluted outdoors, but few studies have been carried out on outdoor transmission of SARS-CoV-2. Additionally, SARS-CoV-2 can be inactivated by ultraviolet radiation in sunlight, and it is likely sensitive to ambient temperature and relative humidity, as well as the presence of atmospheric aerosols that occur in highly polluted areas. Viruses can attach to other particles such as dust and pollution, which can modify the aerodynamic characteristics and increase dispersion. Moreover, people living in areas with higher concentrations of air pollution have been shown to have higher severity of COVID-19 (11). Because respiratory viruses can remain airborne for pro-longed periods before being inhaled by a potential host, studies are needed to characterize the factors leading to loss of infectivity over time in a variety of outdoor environments over a range of conditions.
Given how little is known about the production and air-borne behavior of infectious respiratory droplets, it is difficult to define a safe distance for social distancing. Assuming SARS-CoV-2 virions are contained in submicron aerosols, as is the case for influenza virus, a good comparison is exhaled cigarette smoke, which also contains submicron particles and will likely follow comparable flows and dilution pat-terns. The distance from a smoker at which one smells cigarette smoke indicates the distance in those surroundings at which one could inhale infectious aerosols. In an enclosed room with asymptomatic individuals, infectious aerosol concentrations can increase over time. Overall, the probability of becoming infected indoors will depend on the total amount of SARS-CoV-2 inhaled. Ultimately, the amount of ventilation, number of people, how long one visits an indoor facility, and activities that affect air flow will all modulate viral transmission pathways and exposure (10). For these reasons, it is important to wear properly fitted masks in-doors even when 6 ft apart. Airborne transmission could account, in part, for the high secondary transmission rates to medical staff, as well as major outbreaks in nursing facilities. The minimum dose of SARS-CoV-2 that leads to infection is unknown, but airborne transmission through aerosols has been documented for other respiratory viruses including measles, SARS, and chickenpox (4).
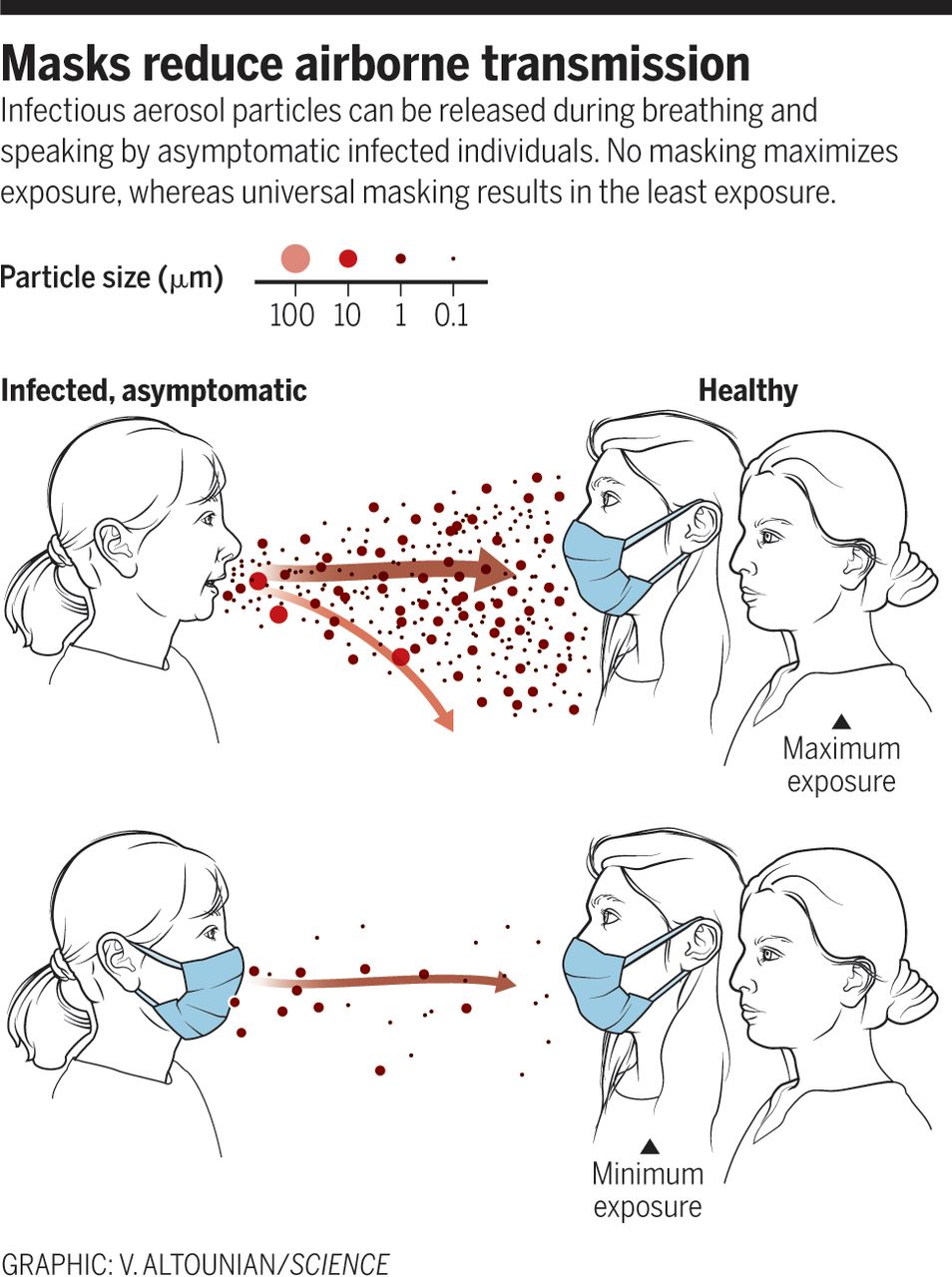
Airborne spread from undiagnosed infections will continuously undermine the effectiveness of even the most vigorous testing, tracing, and social distancing programs. After evidence revealed that airborne transmission by asymptomatic individuals might be a key driver in the global spread of COVID-19, the WHO recommended universal use of face masks. Masks provide a critical barrier, reducing the number of infectious viruses in exhaled breath, especially of asymptomatic people and those with mild symptoms (12) (see the figure). Surgical mask material reduces the likelihood and severity of COVID-19 by substantially reducing airborne viral concentrations (13). Masks also protect uninfected individuals from SARS-CoV-2 aerosols (12, 13). Thus, it is particularly important to wear masks in locations with conditions that can accumulate high concentrations of vi-ruses, such as health care settings, airplanes, restaurants, and other crowded places with reduced ventilation. The aerosol filtering efficiency of different materials, thickness-es, and layers used in properly fitted homemade masks was recently found to be similar to that of the medical masks that were tested (14). Thus, the option of universal masking is no longer held back by shortages.
From epidemiological data, countries that have been most effective in reducing the spread of COVID-19 have implemented universal masking, including Taiwan, Hong Kong, Singapore, and South Korea. In the battle against COVID-19, Taiwan (population 24 million, first COVID-19 case 21 January 2020) did not implement a lockdown during the pandemic, yet maintained a low incidence of 441 cases and 7 deaths (as of 21 May 2020). By contrast, the state of New York (population ~20 million, first COVID case 1 March 2020), had a higher number of cases (353,000) and deaths (24,000). By quickly activating its epidemic response plan that was established after the SARS outbreak, the Taiwanese government enacted a set of proactive measures that successfully prevented the spread of SARS-CoV-2, including setting up a central epidemic command center in January, using technologies to detect and track infected patients and their close contacts, and perhaps most importantly, requesting people to wear masks in public places. The government also ensured the availability of medical masks by banning mask manufacturers from exporting them, implementing a system to ensure that every citizen could acquire masks at reasonable prices, and increasing the production of masks. In other countries, there have been widespread shortages of masks, resulting in most residents not having access to any form of medical mask (15). This striking difference in the availability and widespread adoption of wearing masks likely influenced the low number of COVID-19 cases.
Aerosol transmission of viruses must be acknowledged as a key factor leading to the spread of infectious respiratory diseases. Evidence suggests that SARS-CoV-2 is silently spreading in aerosols exhaled by highly contagious infected individuals with no symptoms. Owing to their smaller size, aerosols may lead to higher severity of COVID-19 because virus-containing aerosols penetrate more deeply into the lungs (10). It is essential that control measures be introduced to reduce aerosol transmission. A multidisciplinary approach is needed to address a wide range of factors that lead to the production and airborne transmission of respiratory viruses, including the minimum virus titer required to cause COVID-19; viral load emitted as a function of droplet size before, during, and after infection; viability of the virus indoors and outdoors; mechanisms of transmission; airborne concentrations; and spatial patterns. More studies of the filtering efficiency of different types of masks are also needed. COVID-19 has inspired research that is already leading to a better understanding of the importance of airborne transmission of respiratory disease.
NOTES:
- Morawska, J. Cao, Environ. Int. 139, 105730 (2020). doi:10.1016/j.envint.2020.105730 Medline
- L. Anderson, P. Turnham, J. R. Griffin, C. C. Clarke, Risk Anal. 40, 902 (2020). doi:10.1111/risa.13500 Medline
- Asadi, N. Bouvier, A. S. Wexler, W. D. Ristenpart, Aerosol Sci. Technol. 54, 635 (2020). doi:10.1080/02786826.2020.1749229
- Tellier, Y. Li, B. J. Cowling, J. W. Tang, BMC Infect. Dis. 19, 101 (2019). doi:10.1186/s12879-019-3707-y Medline
- Mittal, R. Ni, J.-H. Seo, J. Fluid Mech. 10.1017/jfm.2020.330 (2020).
- Chu et al., Clin. Infect. Dis. 10.1093/cid/ciaa410 (2020). Medline
- He et al., Nat. Med. 26, 672 (2020). doi:10.1038/s41591-020-0869-5 Medline
- Liu et al., Nature 10.1038/s41586-020-2271-3 (2020). Medline
- Stadnytskyi, C. E. Bax, A. Bax, P. Anfinrud, Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.2006874117 (2020). Medline
- Buonanno, L. Stabile, L. Morawska, Environ. Int. 141, 105794 (2020). doi:10.1016/j.envint.2020.105794 Medline
- Conticini, B. Frediani, D. Caro, Environ. Pollut. 261, 114465 (2020). doi:10.1016/j.envpol.2020.114465 Medline
- H. L. Leung et al., Nat. Med. 26, 676 (2020). doi:10.1038/s41591-020-0843-2 Medline
- F.-W. Chan et al., Clin. Infect. Dis. 10.1093/cid/ciaa325 (2020). Medline
- Konda et al., ACS Nano 10.1021/acsnano.0c03252 (2020). Medline
- C. Leung, T. H. Lam, K. K. Cheng, Lancet 395, 945 (2020). doi:10.1016/S0140-6736(20)30520-1 Medline
ACKNOWLEDGMENTS:
The authors thank S. Strathdee, D. Petras, and L. Marr for helpful discussions. K.A.P. is supported by the NSF Center for Aerosol Impacts on Chemistry of the Environment (CHE1801971). R.T.S. is supported by the National Institute of Allergy and Infectious Diseases (R01 AI131424). C.C.W. is supported by the Ministry of Science and Technology (MOST 108-2113-M-110-003) and the Higher Education Sprout Project of the Ministry of Education, Taiwan, ROC.
Download article as a PDF File: Reducing Transmission SARS-COV-2
Go to Original – sciencemag.org
Tags: COVID-19, China, Community, Compassion, Coronavirus, Economy, Empathy, Environment, Health, Lockdown, Masks, Pandemic, Public Health, Research, Science, Science and Medicine, Semen, Sharing, Sperm, Trade, United Nations, WHO, World
DISCLAIMER: The statements, views and opinions expressed in pieces republished here are solely those of the authors and do not necessarily represent those of TMS. In accordance with title 17 U.S.C. section 107, this material is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. TMS has no affiliation whatsoever with the originator of this article nor is TMS endorsed or sponsored by the originator. “GO TO ORIGINAL” links are provided as a convenience to our readers and allow for verification of authenticity. However, as originating pages are often updated by their originating host sites, the versions posted may not match the versions our readers view when clicking the “GO TO ORIGINAL” links. This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of environmental, political, human rights, economic, democracy, scientific, and social justice issues, etc. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes. For more information go to: http://www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted material from this site for purposes of your own that go beyond ‘fair use’, you must obtain permission from the copyright owner.
One Response to “Reducing Transmission of SARS-CoV-2”
Read more
Click here to go to the current weekly digest or pick another article:
COVID19 - CORONAVIRUS:
This is obvious, but “universal masking” is overdoing the job.
People walking around far from others, sitting alone in their own cars, walking in the forest with a few friends, always at least a metre from others (NB why use the US∕UK old terms of feet???) or in uncrowded outdoor markets keeping their distance do not need to have masks on all the time. However, those who are ill, or may be infected or suspect so, should keep away from others if they can or OF COURSE then wear a mask and keep their distance. Asymptomatic people (assuming they do not know they are infected ) need to be the most vigilant, and few figures except from Taiwan Island have been provided to indicate how early and how long they are likely to infect others by droplets.
.