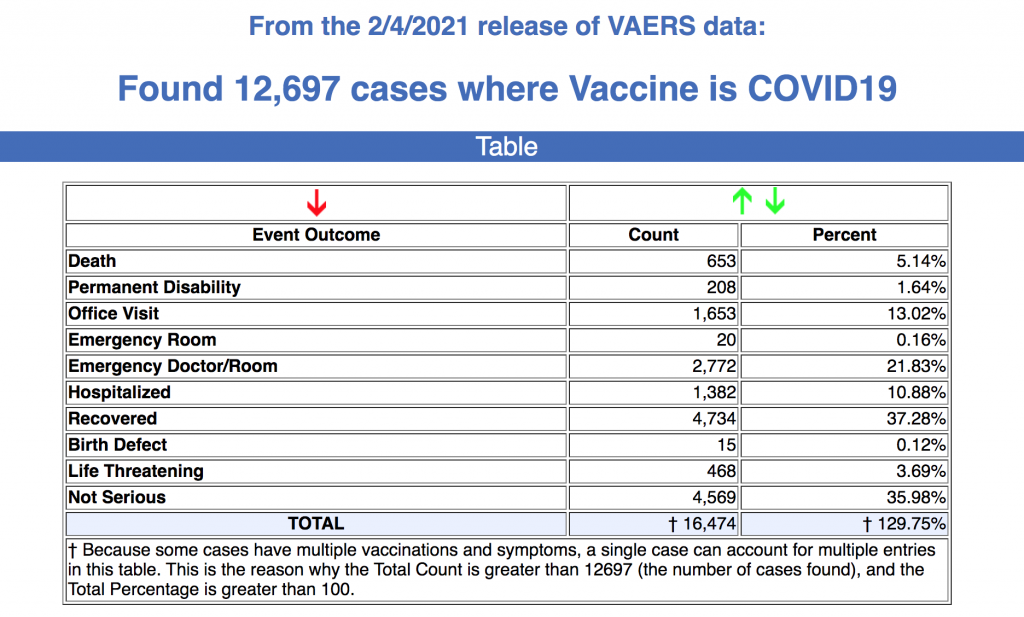
VAERS is the primary mechanism for reporting adverse vaccine reactions in the U.S. Reports submitted to VAERS require further investigation before confirmation can be made that the reported adverse event was caused by the vaccine.
As of Feb. 10, about 44.77 million people in the U.S. had received one or both doses of a COVID vaccine. So far, only the Pfizer and Moderna vaccines have been granted Emergency Use Authorization in the U.S. by the U.S. Food and Drug Administration (FDA). By the FDA’s own definition, the vaccines are still considered experimental until fully licensed.
According to the latest data, 602 of the 653 reported deaths were in the U.S, and 137 of the deaths were related to cardiac disorder. Fifty-three percent of those who died were male, 44% were female, the remaining death reports did not include the gender of the deceased. The average age of those who died was 77, the youngest reported death was of a 23-year-old. The Pfizer vaccine was taken by 58% of those who died, while the Moderna vaccine was taken by 41%.
As or Feb. 4, there had been 163 cases of Bell’s Palsy reported and 775 reports of anaphylaxis.
As The Defender reported today, the CDC is investigating the Feb. 8 death of a 36-year-old doctor in Tennessee who died about a month after receiving the second dose of a COVID vaccination. According to news reports, Dr. Barton Williams died from the adult form of multisystem inflammatory syndrome (MIS-A), a condition caused when the immune system attacks the body resulting in multi-system organ failure. New reports attributed the death to a reaction to an asymptomatic case of COVID, although Williams never tested positive for the virus.
On Feb. 8, Fox5 reported the death of a man in his 70s who collapsed and died Feb. 7 as he was leaving the Javits Center in Manhattan about 25 minutes after receiving a COVID vaccination.
On Feb. 7, a local Villa Hills, Kentucky news site reported on the deaths of two nuns following a “COVID-19 outbreak” that occurred two days after the nuns were vaccinated. Prior to beginning the vaccination program, there had been no cases of COVID at the monastery, which has been shut down to visitors during the pandemic. After vaccinations began, 28 of the women had tested positive for COVID as of Feb. 7.
The clinical trials suggested that almost all the benefits of COVID vaccination and the vast majority of injuries were associated with the second dose.
The Defender also reported this week that according to the New York Times, several doctors now link the Pfizer and Moderna COVID vaccines to immune thrombocytopenia (ITP), a condition that develops when the immune system attacks platelets (blood component essential for clotting) or the cells that create them. The Times article featured two women who are recovering from ITP after being vaccinated. Last month, Dr. Gregory Michaels died from ITP two weeks after he got the Pfizer vaccine.
While the VAERS database numbers may seem sobering, according to a U.S. Department of Health and Human Services study, the actual number of adverse events is likely significantly higher. VAERS is a passive surveillance system that relies on the willingness of individuals to submit reports voluntarily.
According to the VAERS website, healthcare providers are required by law to report to VAERS:
- Any adverse event listed in the VAERS Table of Reportable Events Following Vaccination that occurs within the specified time period after vaccination.
- An adverse event listed by the vaccine manufacturer as a contraindication to further doses of the vaccine.
The CDC says healthcare providers are strongly encouraged to report:
- Any adverse event that occurs after the administration of a vaccine licensed in the United States, whether or not it is clear that a vaccine caused the adverse event.
- Vaccine administration errors.
However, “within the specified time” means that reactions occurring outside that timeframe may not be reported, in addition to reactions suffered hours or days later by people who don’t report those reactions to their healthcare provider.
Vaccine manufacturers are required to report to VAERS “all adverse events that come to their attention.”
Historically, fewer than fewer than 1% of adverse events have ever been reported to VAERS, a system that Children’s Health Defense has previously referred to as an “abject failure,” including in a December 2020 letter to Dr. David Kessler, former FDA director and now co-chair of the COVID-19 Advisory Board and President Biden’s version of Operation Warp Speed.
A critic familiar with VAERS’ shortcomings bluntly condemned VAERS in The BMJ as “nothing more than window dressing, and a part of U.S. authorities’ systematic effort to reassure/deceive us about vaccine safety.”
CHD is calling for complete transparency. The children’s health organization is asking Kessler and the federal government to release all of the data from the clinical trials and suspend COVID-19 vaccine use in any group not adequately represented in the clinical trials, including the elderly, frail and anyone with comorbidities.
CHD is also asking for full transparency in post-marketing data that reports all health outcomes, including new diagnoses of autoimmune disorders, adverse events and deaths from COVID vaccines.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.